IMIZOL- imidocarb dipropionate injection, solution
Imizol by
Drug Labeling and Warnings
Imizol by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp., Vet Pharma Friesoythe GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
SPL UNCLASSIFIED SECTION
129471 R1
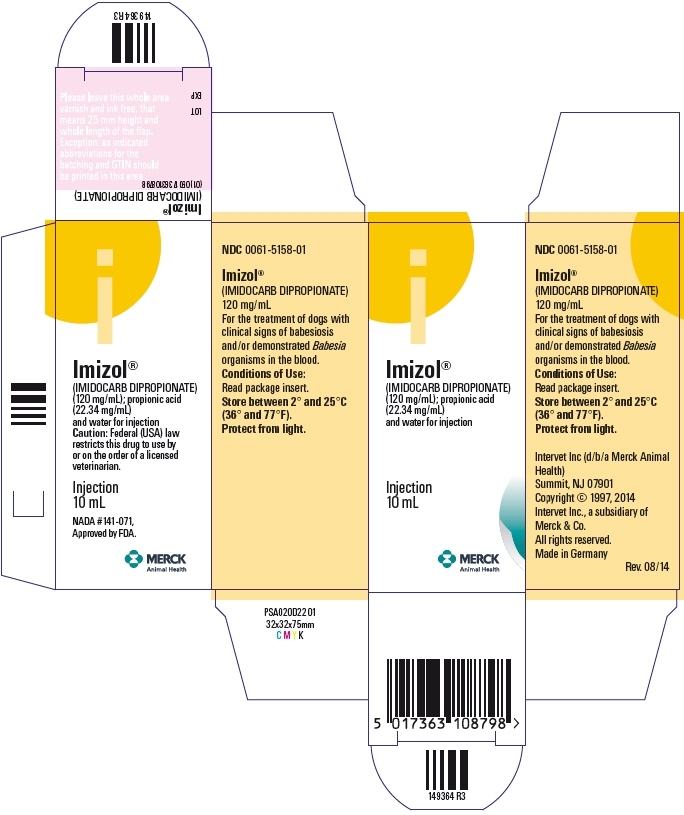
NADA #141-071, Approved by FDA.
NDC: 0061-5158-01Each mL contains 120 mg of imidocarb dipropionate.
Sterile solution for intramuscular or subcutaneous injection. - SPL UNCLASSIFIED SECTION
-
DESCRIPTION:
IMIZOL (imidocarb dipropionate) is a sterile solution containing 120 mg/mL of imidocarb dipropionate suitable for intramuscular or subcutaneous administration. Imidocarb is chemically described as N,N'-bis[3-(4,5-dihydro-1H-imidazol-2-yl)-phenyl]urea dipropionate and has a molecular weight of 496.6. In addition to the active component, imidocarb dipropionate, the formulation also contains propionic acid (22.34 mg/mL), and water for injection.
- INDICATIONS:
-
DOSAGE AND ADMINISTRATION:
Use intramuscularly or subcutaneously at a rate of 6.6 mg/kg (3mg/lb) body weight. Repeat the dose in two (2) weeks, for a total of two (2) treatments.
IMIZOL® DOSING GUIDE 6.6 mg/kg Body Weight Animal Weight IMIZOL Dosage Animal Weight IMIZOL Dosage 10 lb (4.5 kg) 0.25 mL 60 lb (27.3 kg) 1.50 mL 20 lb (9.1 kg) 0.50 mL 80 lb (36.4 kg) 2.00 mL 30 lb (13.6 kg) 0.75 mL 100 lb (45.5 kg) 2.50 mL 40 lb (18.2 kg) 1.00 mL - WARNINGS
-
PRECAUTION:
MUST NOT BE ADMINISTERED INTRAVENOUSLY. The safety and effectiveness of imidocarb have not been determined in puppies or in breeding, lactating, or pregnant animals. Risk versus benefit should be considered before using this drug in dogs with impaired lung, liver, or kidney function.
Do not use this product simultaneously with exposure to cholinesterase-inhibiting drugs, pesticides, or chemicals.
-
ADVERSE EFFECTS:
Adverse effects commonly seen are pain during injection and mild cholinergic signs such as salivation, nasal drip, or brief episodes of vomiting. Other effects seen less frequently are panting, restlessness, diarrhea, and mild injection site inflammation lasting one to several days. Rarely, injection site ulceration occurs, but the lesion is not resistant to healing.
If sever cholinergic signs occur, they may be reversed with atropine sulfate.
To report an adverse reaction, product-related problem, or human exposure, please call Merck Animal Health Technical Services at 1-800-224-5318. To obtain a copy of the Material Safety Data Sheet (MSDS), call 1-800-770-8878. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth/.
-
TOXICOLOGY:
IMIZOL solution was administered subcutaneously to four groups of 5 dogs at 2.2, 5.5, 7.7, or 9.9 mg/kg. The treatment was repeated 2 weeks later. There were no effects attributed to IMIZOL on body temperature, body weight, hematology, most clinical chemistries or gross pathology. At 9.9 mg/kg there was a slight increase in serum alanine aminotransferase (ALT, SGPT) and arginine aminotransferase (AST, SGOT) indicative of mild liver injury. Other effects noted were pain on injection, injection site swelling, and vomiting. Two of the injection sites ulcerated but healed readily without complication.
In a 90-day toxicity study, imidocarb was given orally to three groups of 8 dogs at the rate of 5, 20, or 80 mg/kg/day. The target organs of toxicity were liver and intestines. These results may have been influenced by the oral dosing route. In a pharmacokinetic study by Abdullah et al (1984)1, imidocarb was administered to dogs intravenously at a dose of 4 mg/kg. One of 13 dogs died. The target organs of toxicity in this dog were lungs and kidneys, and some changes were noted in the liver and spleen.
The toxic syndrome involves lethargy, weakness, and anorexia, with possible signs of gastrointestinal, liver, kidney, and lung dysfunction.
-
PHARMACODYNAMICS:
The pharmacodynamics of imidocarb were studied in various species as described by Rao et al (1980)2. The study suggests that there is a potential for adverse reactions mediated by the autonomic nervous system and especially through anticholinesterase mechanisms. Clinical experience in dogs at therapeutic dosages of less than 10 mg/kg body weight given intramuscularly or subcutaneously has established a pattern of adverse reactions. These reactions in descending order of frequency are: salivation, vomiting, and occasionally diarrhea.
- HOW SUPPLIED
- REFERENCES:
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
IMIZOL
imidocarb dipropionate injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5158 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Imidocarb Dipropionate (UNII: ZSM1M03SHC) (Imidocarb - UNII:8USS3K0VDH) Imidocarb Dipropionate 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5158-01 1 in 1 CARTON 1 10 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141071 11/07/1997 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations Vet Pharma Friesoythe GmbH 341934053 MANUFACTURE
Trademark Results [Imizol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IMIZOL 72451713 0974844 Live/Registered |
BURROUGHS WELLCOME CO. 1973-03-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.