WINCO MEDICATED LIP BALM- petrolatum stick
Winco Medicated Lip Balm by
Drug Labeling and Warnings
Winco Medicated Lip Balm by is a Otc medication manufactured, distributed, or labeled by Winco, OraLabs. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
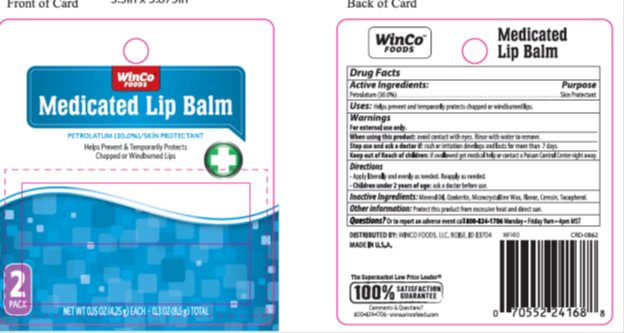
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
- Warnings
- Directions
- Inactive Ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
WINCO MEDICATED LIP BALM
petrolatum stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 67091-251 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 30.0 mg in 1 g Inactive Ingredients Ingredient Name Strength LIGHT MINERAL OIL (UNII: N6K5787QVP) 39 mg in 1 g MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) 3.00 mg in 1 g CERESIN (UNII: Q1LS2UJO3A) 24 mg in 1 g Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67091-251-30 1 g in 1 CONTAINER; Type 0: Not a Combination Product 01/02/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 01/02/2015 Labeler - Winco (056098817) Registrant - OraLabs (801824756) Establishment Name Address ID/FEI Business Operations OraLabs 801824756 MANUFACTURE(67091-251) , LABEL(67091-251)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.