BRIMONIDINE by Padagis Israel Pharmaceuticals Ltd BRIMONIDINE gel
BRIMONIDINE by
Drug Labeling and Warnings
BRIMONIDINE by is a Prescription medication manufactured, distributed, or labeled by Padagis Israel Pharmaceuticals Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BRIMONIDINE TOPICAL GEL safely and effectively. See full prescribing information for BRIMONIDINE TOPICAL GEL.
BRIMONIDINE topical gel
Initial U.S. Approval: 1996INDICATIONS AND USAGE
Brimonidine topical gel, 0.33% is an alpha adrenergic agonist indicated for the topical treatment of persistent (nontransient) facial erythema of rosacea in adults 18 years of age or older. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Gel, 0.33%; Each gram of gel contains 5 mg of brimonidine tartrate, equivalent to 3.3 mg of brimonidine free base. (3)
CONTRAINDICATIONS
Known hypersensitivity to any component of brimonidine topical gel (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
In controlled clinical trials with brimonidine topical gel the most common adverse reactions (incidence ≥ 1%) included erythema, flushing, skin burning sensation, and contact dermatitis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Padagis® at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Potentiation of Vascular Insufficiency
5.2 Severe Cardiovascular Disease
5.3 Serious Adverse Reactions Following Ingestion of Brimonidine Topical Gel
5.4 Systemic Adverse Reactions of Alpha 2-adrenergic agonists
5.5 Local Vasomotor Adverse Reactions
5.6 Hypersensitivity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Anti-hypertensives/Cardiac Glycosides
7.2 CNS Depressants
7.3 Monoamine Oxidase Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Apply a pea-sized amount once daily to each of the five areas of the face: central forehead, chin, nose, each cheek. Brimonidine topical gel should be applied smoothly and evenly as a thin layer across the entire face avoiding the eyes and lips.
Wash hands after applying brimonidine topical gel.
Brimonidine topical gel is for topical use only and not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Potentiation of Vascular Insufficiency
Brimonidine topical gel should be used with caution in patients with depression, cerebral or coronary insufficiency, Raynaud’s phenomenon, orthostatic hypotension, thrombangiitis obliterans, scleroderma, or Sjögren’s syndrome.
5.2 Severe Cardiovascular Disease
Alpha-2 adrenergic agonists can lower blood pressure. Brimonidine topical gel should be used with caution in patients with severe or unstable or uncontrolled cardiovascular disease.
5.3 Serious Adverse Reactions Following Ingestion of Brimonidine Topical Gel
Two young children of a subject in a clinical trial experienced serious adverse reactions following accidental ingestion of brimonidine topical gel. Adverse reactions experienced by one or both children included lethargy, respiratory distress with apneic episodes (requiring intubation), sinus bradycardia, confusion, psychomotor hyperactivity, and diaphoresis. Both children were hospitalized overnight and discharged the following day without sequelae.
Keep brimonidine topical gel out of the reach of children.
5.4 Systemic Adverse Reactions of Alpha 2-adrenergic agonists
Postmarketing cases of bradycardia, hypotension (including orthostatic hypotension) and dizziness have been reported. Some cases required hospitalization. Some cases involved application of brimonidine topical gel in unapproved dosing regimens and for unapproved indications, including the application of brimonidine topical gel following laser procedures.
Avoid applying brimonidine topical gel to irritated skin or open wounds.
5.5 Local Vasomotor Adverse Reactions
Erythema
Some subjects in the clinical trials discontinued use of brimonidine topical gel because of erythema. Some subjects in the clinical trials reported a rebound phenomenon, where erythema was reported to return worse compared to the severity at baseline. Erythema appeared to resolve after discontinuation of brimonidine topical gel [see Adverse Reactions (6.1)].
The treatment effect of brimonidine topical gel may begin to diminish hours after application.
From postmarketing reports, some patients have experienced erythema involving areas of the face that were previously not affected by erythema and in areas (e.g., neck and chest) outside of the treatment sites.
Flushing
Some subjects in the clinical trials discontinued use of brimonidine topical gel because of flushing.
Intermittent flushing occurred in some subjects treated with brimonidine topical gel in the clinical trials. The onset of flushing relative to application of brimonidine topical gel varied, ranging from approximately 30 minutes to several hours [see Adverse Reactions (6.1)]. Flushing appeared to resolve after discontinuation of brimonidine topical gel.
From postmarketing reports, some patients have experienced increased frequency of flushing and/or increased depth of erythema with the flushing. Additionally, some patients reported new onset of flushing.
Pallor and Excessive Whitening
From postmarketing reports, some patients have experienced pallor or excessive whitening at or outside the application site following treatment with brimonidine topical gel.
5.6 Hypersensitivity
Allergic contact dermatitis was reported in the clinical trials for brimonidine topical gel [see Adverse Reactions (6.1)].
Events reported post marketing with the use of brimonidine topical gel include angioedema, throat tightening, tongue swelling, and urticarial [see Adverse Reactions (6.2)]. Institute appropriate therapy and discontinue brimonidine topical gel, if clinically significant hypersensitivity reaction occurs.
-
6 ADVERSE REACTIONS
The following adverse drug reactions are discussed in greater detail in other sections of the label:
- Systemic Adverse Reactions of Alpha-2 Adrenergic Agonists [see Warnings and Precautions (5.4)]
- Local Vasomotor Adverse Reactions [see Warnings and Precautions (5.5)]
- Hypersensitivity [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During clinical trials, 1210 subjects were exposed to brimonidine topical gel. A total of 833 subjects were treated for persistent (nontransient) erythema associated with rosacea, and 330 of those were treated once daily for 29 days in vehicle-controlled trials.
Adverse reactions that occurred in at least 1% of subjects treated with brimonidine topical gel once daily for 29 days and for which the rate for brimonidine topical gel exceeded the rate for vehicle are presented in Table 1.
Table 1 - Adverse Reactions Reported in Clinical Trials by at Least 1% of Subjects Treated for 29 Days
Preferred Term
Brimonidine Topical Gel
(N=330)
n (%)Vehicle Gel
(N=331)
n (%)Subjects with at least one adverse reaction, Number (%) of Subjects
109 (33)
91 (28)
Erythema
12 (4%)
3 (1%)
Flushing
9 (3%)
0
Skin burning sensation
5 (2%)
2 (1%)
Dermatitis contact
3 (1%)
1 (˂1%)
Dermatitis
3 (1%)
1 (˂1%)
Skin warm
3 (1%)
0
Paraesthesia
2 (1%)
1 (˂1%)
Acne
2 (1%)
1 (˂1%)
Pain of skin
2 (1%)
0
Vision blurred
2 (1%)
0
Nasal congestion
2 (1%)
0
Open-label, Long-term Study
An open-label study of brimonidine topical gel when applied once daily for up to one year was conducted in subjects with persistent (nontransient) facial erythema of rosacea. Subjects were allowed to use other rosacea therapies. A total of 276 subjects applied brimonidine topical gel for at least one year. The most common adverse events (≥ 4% of subjects) for the entire study were flushing (10%), erythema (8%), rosacea (5%), nasopharyngitis (5%), skin burning sensation (4%), increased intraocular pressure (4%), and headache (4%).
Allergic contact dermatitis
Allergic contact dermatitis to brimonidine topical gel was reported in approximately 1% of subjects across the clinical development program. Two subjects underwent patch testing with individual product ingredients. One subject was found to be sensitive to brimonidine tartrate, and one subject was sensitive to phenoxyethanol (a preservative).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of brimonidine topical gel. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular disorders: bradycardia, hypotension (including orthostatic hypotension)
Immune system disorders: angioedema, hypersensitivity, lip swelling, swollen tongue, throat tightness, urticaria
Nervous system disorders: dizziness
Skin and subcutaneous disorders: pallor
-
7 DRUG INTERACTIONS
7.1 Anti-hypertensives/Cardiac Glycosides
Alpha-2 agonists, as a class, may reduce blood pressure. Caution in using drugs such as beta-blockers, anti-hypertensives and/or cardiac glycosides is advised.
7.2 CNS Depressants
Although specific drug-drug interactions studies have not been conducted with brimonidine topical gel, the possibility of an additive or potentiating effect with CNS depressants (alcohol, barbiturates, opiates, sedatives, or anaesthetics) should be considered.
7.3 Monoamine Oxidase Inhibitors
Monoamine oxidase (MAO) inhibitors may theoretically interfere with the metabolism of brimonidine and potentially result in an increased systemic side-effect such as hypotension. Caution is advised in patients taking MAO inhibitors which can affect the metabolism and uptake of circulating amines.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B.
There are no adequate and well-controlled studies of brimonidine topical gel in pregnant women. In animal studies, brimonidine crossed the placenta and entered into the fetal circulation to a limited extent. Brimonidine topical gel should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Brimonidine tartrate was not teratogenic when given at oral doses up to 2.5 mg/kg/day in pregnant rats during gestation days 6 through 15 and 5 mg/kg/day in pregnant rabbits during gestation days 6 through 18.
8.3 Nursing Mothers
It is not known whether brimonidine tartrate is excreted in human milk, although in animal studies, brimonidine tartrate has been shown to be excreted in breast milk. Because of the potential for serious adverse reactions from brimonidine topical gel in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Keep brimonidine topical gel out of reach of children. Serious adverse reactions were experienced by two children of a subject in a clinical trial who accidentally ingested brimonidine topical gel [see Warnings and Precautions (5.3)].
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
One hundred and five subjects aged 65 and older were included in clinical trials with brimonidine topical gel. No overall differences in safety or effectiveness were observed between subjects ≥ 65 years of age and younger adult subjects. Clinical studies of brimonidine topical gel did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
-
10 OVERDOSAGE
No information is available on overdose in adults with brimonidine topical gel.
Oral overdoses of other alpha-2 adrenergic agonists have been reported to cause symptoms such as hypotension, asthenia, vomiting, lethargy, sedation, bradycardia, arrhythmias, miosis, apnoea, hypotonia, hypothermia, respiratory depression, and seizure.
Treatment of an oral overdose includes supportive and symptomatic therapy; a patent airway should be maintained.
-
11 DESCRIPTION
Brimonidine Topical Gel, 0.33% contains brimonidine tartrate, an alpha adrenergic agonist.
The molecular formula of brimonidine tartrate is C11H10BrN5 C4H6O6. It has the following structural formula:
Chemically, brimonidine tartrate is 5-Bromo-6-(2-imidazolidinylideneamino) quinoxaline L-tartrate. Brimonidine tartrate has a molecular weight of 442.24 and appears as white to slightly yellowish powder.
Each gram of Brimonidine Topical Gel, 0.33% contains 5 mg of the active ingredient brimonidine tartrate (equivalent to 3.3 mg of brimonidine free base), in a white to light yellow opaque gel composed of the inactive ingredients benzalkonium chloride, carbomer homopolymer type B, glycerin, propylene glycol, purified water, sodium hydroxide, and talc.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Brimonidine is a relatively selective alpha-2 adrenergic agonist. Topical application of brimonidine topical gel may reduce erythema through direct vasoconstriction.
12.3 Pharmacokinetics
Absorption
The absorption of brimonidine from brimonidine topical gel was evaluated in a clinical trial in 24 adult subjects with facial erythema associated with rosacea. All enrolled subjects received once daily topical application of brimonidine topical gel 1 gram to the entire face for 29 days. Pharmacokinetic assessments were performed on Day 1, Day 15, and Day 29. The mean plasma maximum concentration (Cmax) and area under the concentration-time curve (AUC) were highest on Day 15, with Cmax and AUC values (± standard deviation) of 46 ± 62 pg/mL and 417 ± 264 pg.hr/mL, respectively. The systemic drug exposure was slightly lower on Day 29 indicating no further drug accumulation.
Metabolism
Brimonidine is extensively metabolized by the liver.
Excretion
Urinary excretion is the major route of elimination of brimonidine and its metabolites.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 21-month oral (diet) mouse carcinogenicity study and a 24-month oral (diet) rat carcinogenicity study, no drug-related neoplasms were observed in mice at oral doses of brimonidine tartrate up to 2.5 mg/kg/day or in rats at oral doses of brimonidine tartrate up to 1 mg/kg/day.
In a dermal rat carcinogenicity study with brimonidine topical gel, brimonidine tartrate was administered to Wistar rats at topical doses of 0.9 (0.03% gel), 1.8 (0.06% gel), and 5.4 mg/kg/day (0.18% gel) in males and 5.4 (0.18% gel), 30 (1% gel) during Days 1-343/10.8 (0.36% gel) thereafter, and 60 (2% gel) during Days 1-343/21.6 mg/kg/day (0.72% gel) thereafter in females once daily for 24 months. No drug-related neoplasms were observed in this study.
In a 12-month dermal photo-carcinogenicity study, topical doses of 0% (brimonidine topical gel vehicle), 0.18%, 1% and 2% brimonidine tartrate gel were administered to hairless albino mice once daily, five days per week, with concurrent exposure to simulated sunlight. No drug-related adverse effects were observed in this study. The results of this study suggest that topical treatment with brimonidine topical gel would not enhance photo-carcinogenesis.
Mutagenesis
Brimonidine tartrate was not mutagenic or clastogenic in a series of in vitro and in vivo studies, including the Ames test, a chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells, and three studies in CD1 mice (a host-mediated assay, a cytogenetic study, and a dominant lethal assay).
Impairment of Fertility
Reproduction and fertility studies in rats with brimonidine tartrate demonstrated no adverse effects on male or female fertility at oral doses up to 1 mg/kg/day.
-
14 CLINICAL STUDIES
Brimonidine topical gel was evaluated for the treatment of moderate to severe, persistent (nontransient) facial erythema of rosacea in two randomized, double-blind, vehicle-controlled clinical trials, which were identical in design. The trials were conducted in 553 subjects aged 18 years and older who were treated once daily for 4 weeks with either brimonidine topical gel or vehicle. Overall, 99% of subjects were Caucasian and 76% were female. Baseline disease severity was graded using a 5-point Clinical Erythema Assessment (CEA) scale and a 5-point Patient Self Assessment (PSA) scale, on which subjects scored either “moderate” or “severe” on both scales.
The primary efficacy endpoint in both pivotal trials was 2-grade Composite Success, defined as the proportion of subjects with a 2-grade improvement on both CEA and PSA measured at hours 3, 6, 9, and 12 on Day 29. Table 2 presents the efficacy results. In addition to Day 29, efficacy was evaluated on Day 15 and Day 1, and the results are presented in Figures 1 and 2 for Studies 1 and 2, respectively.
Table 2: Summary of 2-grade Composite Success on Day 29
Success
Study 1
Study 2
Brimonidine Topical Gel (N=129)
Vehicle Gel (N=131)
Brimonidine Topical Gel (N=148)
Vehicle Gel (N=145)
Hour 3
31%
11%
25%
9%
Hour 6
30%
10%
25%
9%
Hour 9
26%
10%
18%
11%
Hour 12
23%
9%
22%
10%
2-grade Composite Success: 2-grade improvement on CEA and 2-grade improvement on PSA.
Figure 1: 2-grade Composite Success by Hour and Day for Study 1
Figure 2: 2-grade Composite Success by Hour and Day for Study 2
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Brimonidine Topical Gel, 0.33% is a white to light yellow opaque gel, supplied in a laminated tube or pump with a child resistant cap in the following sizes:
30 gram tube NDC 45802-078-94
45 gram tube NDC 45802-078-84
30 gram pump NDC 45802-078-30
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (59°F and 86°F) [See USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use). Patients using brimonidine topical gel should receive the following information and instructions:
- This medication is to be used as directed by the physician.
- It is for external use only.
- Brimonidine topical gel should not be applied to irritated skin or open wounds.
- Avoid contact with the eyes and lips.
- Patients should wash their hands immediately after applying the medication.
- Some patients using brimonidine topical gel may experience erythema, flushing or excessive whitening.
- Patients should report any adverse reactions to their physician.
- Keep out of reach of children.
Manufactured by Padagis®
Yeruham, Israelwww.padagis.com
Rev 07-24
7M15B RC PH3
-
PATIENT INFORMATION
PATIENT INFORMATION
Brimonidine (bri-MOE-ni-deen) Topical Gel, 0.33%
Important information: Brimonidine topical gel is for use on the face only. Do not use brimonidine topical gel in your eyes, mouth, or vagina.
Keep brimonidine topical gel out of the reach of children.
If anyone, especially a child, accidentally swallows brimonidine topical gel, they may have serious side effects and need to be treated in a hospital. Get medical help right away if you, a child, or anyone else swallows brimonidine topical gel and has any of these symptoms:
lack of energy, trouble breathing or stops breathing, a slow heart beat, confusion, sweating, restlessness, muscle spasms, or twitching.
What is brimonidine topical gel?
Brimonidine topical gel is a prescription medicine that is used on your skin (topical) to treat facial redness due to rosacea that does not go away (persistent) in adults who are 18 years of age or older.
It is not known if brimonidine topical gel is safe and effective in children.
Who should not use brimonidine topical gel?
Do not use brimonidine topical gel if you have had a serious allergic reaction to any of the ingredients in brimonidine topical gel. See the end of this Patient Information leaflet for a list of ingredients in brimonidine topical gel. See “What are the possible side effects of brimonidine topical gel?”
What should I tell my doctor before using brimonidine topical gel?
Before using brimonidine topical gel, tell your doctor about all of your medical conditions including if you:
have depression
have heart or blood vessel problems
have dizziness or blood pressure problems
have problems with blood circulation or have had a stroke
have dry mouth or Sjögren’s Syndrome
have skin tightening or scleroderma
have Raynaud’s phenomenon
have irritated skin or open sores
plan to have any laser procedures
are pregnant or plan to become pregnant. It is not known if brimonidine topical gel will harm your unborn baby.
are breastfeeding. It is not known if brimonidine passes into your breast milk. You and your doctor should decide if you will use brimonidine topical gel or breastfeed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, skin products, vitamins, and herbal supplements. Using brimonidine topical gel with certain other medicines may affect each other and can cause serious side effects.
How should I use brimonidine topical gel?
See the detailed Instructions for Use that comes with your brimonidine topical gel tube or pump for information about how to apply brimonidine topical gel correctly.
Use brimonidine topical gel exactly as your doctor tells you. Do not use more brimonidine topical gel than prescribed. Call your doctor if you are not sure.
You should not apply brimonidine topical gel to irritated skin or open wounds.
Brimonidine topical gel is for use on your skin only. Do not use brimonidine topical gel in your eyes, mouth, or vagina. Avoid contact with your lips and eyes.
What are the possible side effects of brimonidine topical gel?
Brimonidine topical gel may cause serious side effects, including:
See “Important information” at the beginning of this Patient Information leaflet.
Problems with blood circulation. People who use brimonidine topical gel can have problems with blood circulation, including a slow heart rate, low blood pressure, and dizziness. These problems may sometimes be serious and lead to hospitalization. See “What should I tell my doctor before using brimonidine topical gel?”
Serious allergic (hypersensitivity) reactions have happened in people who use brimonidine topical gel. Stop using brimonidine topical gel and go to the nearest hospital emergency room right away if you have any of the following signs and symptoms of a serious allergic reaction including:
◦ swelling of your face, lips, tongue, or throat
◦ hives
◦ trouble breathing
The most common side effects of brimonidine topical gel include:
redness
flushing
burning sensation of the skin
skin reactions (contact dermatitis).
Skin redness is common after applying brimonidine topical gel, and may be worse than before you applied it. You may also develop redness on areas of your face that were not affected by rosacea, as well as on your neck and chest.
Skin flushing is common and may happen off and on after applying brimonidine topical gel. In some cases, the flushing may be new, may happen more often, or you may have increased redness with flushing.
Pale colored skin or very white skin (excessive whitening) can happen at or outside the treated area.
Tell your doctor if you get skin redness, flushing, and pale colored skin that is uncomfortable for you.
These are not all the possible side effects of brimonidine topical gel.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of brimonidine topical gel
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or doctor for information about brimonidine topical gel that is written for health professionals. Do not use brimonidine topical gel for a condition for which it was not prescribed. Do not give brimonidine topical gel to other people, even if they have the same symptoms that you have. It may harm them.
What are the ingredients in brimonidine topical gel?
Active ingredient: brimonidine
Inactive ingredients: benzalkonium chloride, carbomer homopolymer type B, glycerin, propylene glycol, purified water, sodium hydroxide, talc.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured by Padagis®, Yeruham, Israel
www.padagis.comRev 07-24
7M15B RC PH3
-
Instructions for Use
Instructions for Use
Brimonidine (bri-MOE-ni-deen) Topical Gel, 0.33%
Pump
Important: Brimonidine topical gel is for use on the face only. Do not use brimonidine topical gel in your eyes, mouth, or vagina.
Keep brimonidine topical gel out of the reach of children.
If anyone, especially a child, accidentally swallows brimonidine topical gel, they may have serious side effects and need to be treated in a hospital. Get medical help right away if you, a child, or anyone else swallows brimonidine topical gel and has any of these symptoms:
- lack of energy, trouble breathing or stops breathing, a slow heart beat, confusion, sweating, restlessness, muscle spasms, or twitching.
Read and follow the steps below so that you use your brimonidine topical gel pump correctly:
- 1.
Push the cap down and turn it counter-clockwise until the cap can be removed. See Figures A and B.
The sticker will break when opening for the first time.
Note: when the cap is removed, the pump is not child-resistant.
Before the first use, prime the pump by pressing down several times until the medicine is dispensed onto your fingertip.
- 2. To apply brimonidine topical gel to your face, dispense a pea-sized amount of brimonidine topical gel from the pump onto your fingertip. See Figure C.
- 3. Apply a pea-sized amount of brimonidine topical gel onto each of the five areas of your face (forehead, chin, nose, each cheek) 1 time each day. You will use a total of 5 pea-sized amounts of brimonidine topical gel. Spread the gel smoothly and evenly in a thin layer over your face. Avoid contact with your eyes and lips. Do not apply brimonidine topical gel to irritated skin or open wounds.
- 4. To close your brimonidine topical gel pump, place the cap back on the pump. Push down and turn the cap to the right (clockwise) until it stops. This pump is child-resistant again. See Figure D.
- 5. Wash your hands right away after applying brimonidine topical gel.
How should I store brimonidine topical gel?
- Store brimonidine topical gel at room temperature between 68°F to 77°F (20°C to 25°C).
Keep brimonidine topical gel and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by Padagis®, Yeruham, Israel
www.padagis.comRev 07-24
7M15B RC PH3
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 45802-078-30
Rx Only
Brimonidine Topical Gel, 0.33%
For Topical Use Only
Keep Out of Reach of Children
Not for oral, ophthalmic or intravaginal use.
PUMP
NET WT 30 g
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
-
INGREDIENTS AND APPEARANCE
BRIMONIDINE
brimonidine gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 45802-078 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRIMONIDINE TARTRATE (UNII: 4S9CL2DY2H) (BRIMONIDINE - UNII:E6GNX3HHTE) BRIMONIDINE TARTRATE 5 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) TALC (UNII: 7SEV7J4R1U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 45802-078-30 1 in 1 CARTON 01/01/2023 1 30 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209158 01/01/2023 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
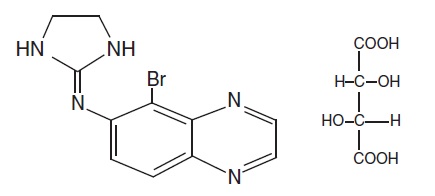
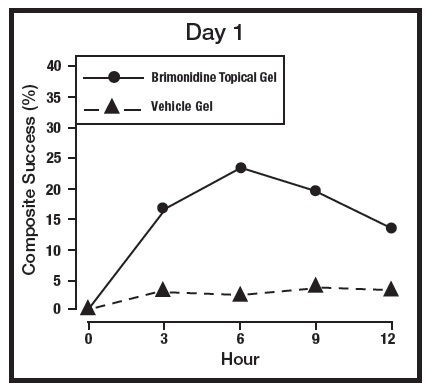
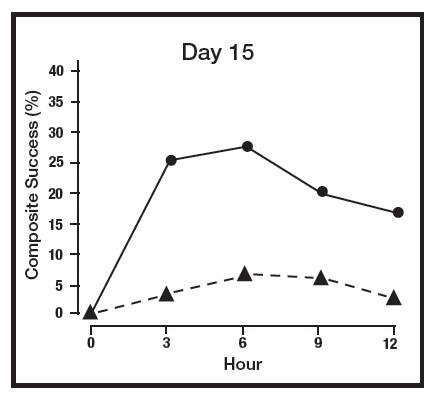
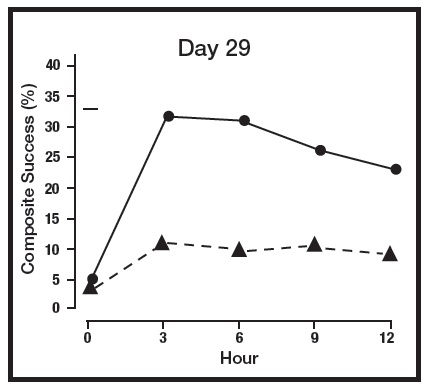
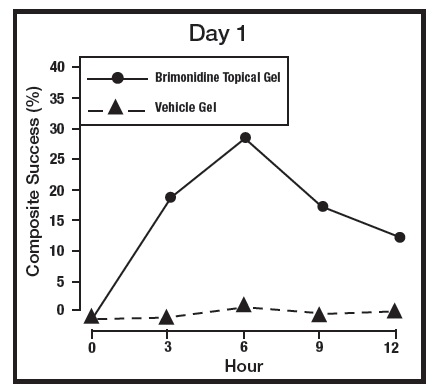
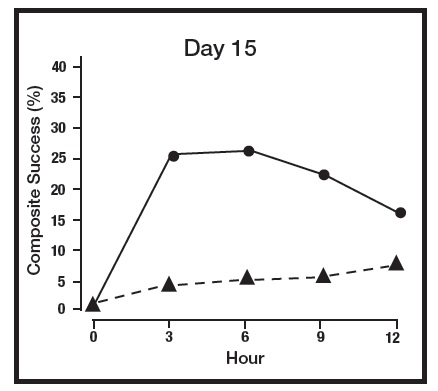
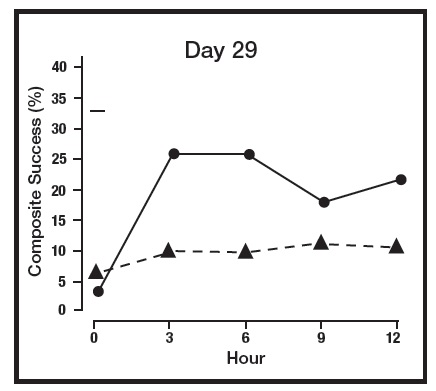
 Figure A
Figure A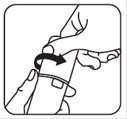 Figure B
Figure B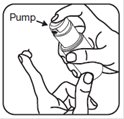 Figure C
Figure C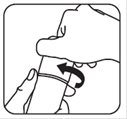 Figure D
Figure D
