VOYXACT- sibeprenlimab injection
VOYXACT by
Drug Labeling and Warnings
VOYXACT by is a Prescription medication manufactured, distributed, or labeled by Otsuka America Pharmaceutical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VOYXACT safely and effectively. See full prescribing information for VOYXACT.
VOYXACT® (sibeprenlimab-szsi) injection, for subcutaneous use
Initial U.S. Approval: 2025INDICATIONS AND USAGE
VOYXACT is an A Proliferation Inducing Ligand (APRIL) blocker, indicated to reduce proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk for disease progression. (1)
This indication is approved under accelerated approval based on reduction of proteinuria. It has not been established whether VOYXACT slows kidney function decline over the long-term in patients with IgAN. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory clinical trial. (1)
DOSAGE AND ADMINISTRATION
Recommended dosage: 400 mg injected subcutaneously once every 4 weeks. (2.1)
DOSAGE FORMS AND STRENGTHS
Injection: 400 mg/2 mL (200 mg/mL) in a single-dose prefilled syringe. (3)
CONTRAINDICATIONS
Serious hypersensitivity to sibeprenlimab-szsi or any excipients in VOYXACT. (4)
WARNINGS AND PRECAUTIONS
- Immunosuppression and Increased Risk of Infections: Before initiating VOYXACT, assess for active infections. During treatment, monitor for signs and symptoms of infection. (5.1)
- Immunosuppression and Immunization Risks: Live vaccines not recommended within 30 days prior to initiation of VOYXACT or during treatment. (5.2)
ADVERSE REACTIONS
Most common adverse reactions are upper respiratory tract infection and injection site erythema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Otsuka America Pharmaceutical, Inc. at 1-800-438-9927 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Missed Dose
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Immunosuppression and Increased Risk of Infections
5.2 Immunosuppression and Immunization Risks
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VOYXACT is indicated to reduce proteinuria in adults with primary immunoglobulin A nephropathy (IgAN) at risk for disease progression.
This indication is approved under accelerated approval based on reduction of proteinuria. It has not been established whether VOYXACT slows kidney function decline over the long-term in patients with IgAN. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory clinical trial.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of VOYXACT is 400 mg administered by subcutaneous injection once every 4 weeks.
2.2 Missed Dose
If a scheduled dose of VOYXACT is missed, administer the missed dose as soon as possible and then resume dosing every 4 weeks thereafter.
2.3 Preparation and Administration
VOYXACT is intended for patient self-administration or for administration by a caregiver. Provide proper training to patients and/or caregivers on the administration of VOYXACT prior to use according to the "Instructions for Use".
Visually inspect the prefilled syringe for particulate matter and discoloration. The solution should be clear to opalescent and colorless to yellow. Do not use the prefilled syringe if the solution contains visible particulate matter, is cloudy or discolored (other than clear to opalescent, colorless to yellow).
Allow VOYXACT prefilled syringe to come to room temperature up to 77°F (25°C) for 15 to 30 minutes before giving an injection. Keep VOYXACT prefilled syringe in the original carton to protect it from light. Once VOYXACT prefilled syringe has reached room temperature, do not return it to the refrigerator. Do not use VOYXACT if it has been at room temperature for 7 days or longer.
Administer VOYXACT by subcutaneous injection only. Inject into the front of the thigh or abdomen. The back of the upper arm can also be used as an injection site if administered subcutaneously by a caregiver. Do not inject into the same site used for the previous injection, or into moles, scars, bruises or areas where the skin is tender, damaged, red, scaly, or hard.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Immunosuppression and Increased Risk of Infections
VOYXACT suppresses the immune system by reducing antibody production, which may increase the risk of infections. Patients with chronic or recurring infections may have an increased risk of serious infection. In clinical trials, infections occurred in 49% of patients treated with VOYXACT compared with 45% of patients treated with placebo.
Before initiating VOYXACT, assess patients for active infections. During treatment, monitor patients for signs and symptoms of infection. If a serious infection develops, consider interrupting VOYXACT until the infection is controlled.
There are limited clinical study data with concomitant use of VOYXACT and systemic immuno-suppressants. Consider the potential for increased immunosuppression when coadministering VOYXACT and immuno-suppressants or when initiating VOYXACT before or after immuno-suppressive therapy.
5.2 Immunosuppression and Immunization Risks
Because of its mechanism of action, VOYXACT may interfere with the immune responses to vaccines and increase the risk of infection from live vaccines. Live vaccines are not recommended within 30 days prior to initiation of VOYXACT or during treatment with VOYXACT as safety has not been established. No data are available on the secondary transmission of infection from persons receiving live vaccines to patients receiving VOYXACT or on the efficacy of immunizations administered while receiving VOYXACT.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Immunosuppression and Increased Risk of Infections [see Warnings and Precautions (5.1)]
- Immunosuppression and Immunization Risks [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of VOYXACT was evaluated in a randomized, double-blind, placebo-controlled, clinical study in patients with IgAN (VISIONARY). The median duration of exposure was 44 weeks in the 259 patients treated with VOYXACT and 48 weeks in the 251 patients administered placebo. The most common adverse reactions (reported in ≥10% of patients treated with VOYXACT and at a higher incidence than placebo) in patients treated with VOYXACT and placebo, respectively, were infections (49% versus 45%) and injection site reactions (24% versus 23%). The most common infection was upper respiratory infection (15% versus 14%), and the most common injection site reaction was injection site erythema (13% versus 12%). Most adverse reactions were reported as mild or moderate in severity and resolved without treatment interruption or discontinuation.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on VOYXACT use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Monoclonal antibodies, such as sibeprenlimab-szsi, can be actively transported across the placenta as pregnancy progresses; therefore, potential effects on a fetus are likely to be greater during the second and third trimester of pregnancy (see Clinical Considerations). In an enhanced prenatal and postnatal development (ePPND) toxicity study, administration of sibeprenlimab-szsi subcutaneously to pregnant monkeys did not result in any adverse effects on embryofetal or postnatal development at exposures approximately 10-times the clinical exposure at the maximum recommended human dose (MRHD) based on area under the curve (AUC) (see Data).
The background risk of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Pregnant women exposed to VOYXACT, or their healthcare providers, should report VOYXACT exposure by calling 1-833-869-9228 or visiting www.VOYXACT.com.
Disease-Associated Maternal and/or Embryo/Fetal Risk
IgA nephropathy is associated with adverse maternal outcomes, including increased rates of cesarean section, pregnancy-induced hypertension, pre-eclampsia and preterm delivery, and adverse fetal/neonatal outcomes, including stillbirth and low birth weight.
Fetal/Neonatal Adverse Reactions
Transport of endogenous IgG antibodies across the placenta increases as pregnancy progresses, and peaks during the third trimester. Therefore, VOYXACT may be present in infants exposed in utero. Consider the potential clinical impact of VOYXACT exposure in infants who were exposed to VOYXACT in utero.
Animal Data
In an ePPND toxicity study, subcutaneous administration of sibeprenlimab-szsi once every two weeks to pregnant cynomolgus monkeys from gestation Day 20 through delivery (includes the period of organogenesis) did not result in any adverse effect on embryofetal or postnatal development at the single tested dose of 101 mg/kg, which provided an approximately 10-fold margin to the clinical exposure at the MRHD based on AUC.
8.2 Lactation
Risk Summary
There are no data on the presence of sibeprenlimab-szsi in human milk, the effects of sibeprenlimab-szsi on the breastfed infant, or the effects of sibeprenlimab-szsi on milk production. Endogenous maternal IgG and monoclonal antibodies are transferred into human milk. The effects of local gastrointestinal exposure on sibeprenlimab-szsi in the breastfed infant are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for VOYXACT and any potential adverse effects on the breastfed child from VOYXACT or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of VOYXACT in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of VOYXACT did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger adult patients.
No clinically meaningful differences in the pharmacokinetics of VOYXACT were observed in patients aged 65 and over compared to younger adult patients [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
VOYXACT contains sibeprenlimab-szsi, an A Proliferation Inducing Ligand (APRIL) blocker and humanized immunoglobulin G2 (IgG2) monoclonal antibody produced by Chinese Hamster Ovary (CHO) cells.
The approximate molecular weight of sibeprenlimab-szsi is 146 kDa.
VOYXACT (sibeprenlimab-szsi) injection is a sterile, preservative-free, clear to opalescent, colorless to yellow solution in a single-dose prefilled syringe for subcutaneous use.
Each 2 mL prefilled syringe delivers 400 mg sibeprenlimab-szsi and the inactive ingredients: arginine (17.6 mg), glutamic acid (14.8 mg), histidine (4.34 mg), L-histidine hydrochloride monohydrate (4.62 mg), polysorbate 80 (0.40 mg), sorbitol (36.4 mg), and Water for Injection, USP. The pH is 6.2.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
VOYXACT binds to APRIL with a dissociation constant (KD) of 0.95 pM, which blocks signaling at the B cell maturation antigen (BCMA) and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) receptors. Inhibition of APRIL results in reduced levels of serum galactose-deficient immunoglobulin A1 (Gd-IgA1), which is implicated in the pathogenesis of IgAN.
12.2 Pharmacodynamics
In healthy subjects administered a single 400 mg subcutaneous VOYXACT dose, serum levels of APRIL were reduced by >90% by Day 3 and the reduction was maintained until Day 42.
In IgAN patients treated with VOYXACT every four weeks in the VISIONARY Study, APRIL suppression > 90% was observed by Week 4 and was sustained throughout treatment. Serum Gd-IgA1, IgA, IgG, and IgM levels decreased within 4 weeks, reached a plateau by Week 24, and by Week 48 mean serum levels were reduced from baseline by 67% for Gd-IgA1, 69% for IgA, 35% for IgG, and 75% for IgM.
12.3 Pharmacokinetics
Following 400 mg doses of VOYXACT administered subcutaneously every 4 weeks to patients with IgAN, steady-state is reached by 20 weeks of dosing.
Absorption
Following a single subcutaneous dose of 400 mg VOYXACT in healthy subjects, the median time to reach peak concentration is 8 days.
The absolute bioavailability of sibeprenlimab-szsi is approximately 92% and there is no difference in relative bioavailability following administration to the abdomen, thigh, or arm.
Distribution
The volume of distribution is 4 L.
Elimination
Following a single 400 mg VOYXACT dose, the mean terminal half-life is 9.3 days. The mean apparent clearance of sibeprenlimab-szsi following 400 mg doses every 4 weeks is 206 mL/day.
Metabolism
As an IgG2 monoclonal antibody, sibeprenlimab-szsi is expected to be degraded by proteolytic enzymes via catabolic pathways in the same manner as endogenous IgG.
Specific Populations
No dedicated studies were conducted to evaluate the effects of renal impairment and hepatic impairment on the pharmacokinetics of sibeprenlimab-szsi.
Population pharmacokinetic analysis did not identify any clinically relevant differences in the pharmacokinetics of sibeprenlimab-szsi based on sex, age, weight, race, and mild to moderate renal impairment (eGFR: 30 to 89 mL/min).
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the study described below with the incidence of ADA in other studies, including those of sibeprenlimab-szsi.
During the treatment period in the VISIONARY Study, 88 of 256 (34%) evaluable patients treated with VOYXACT developed ADA. Among the 88 patients who tested positive for ADA, 21 patients (23.9%) developed ADA that had neutralizing activity. Sibeprenlimab-szsi exposure in patients with IgAN who developed ADA was ~ 40% lower than in patients with undetectable ADA over the treatment period. The reduction in urine protein/creatinine ratio based on 24-hour urine collections (uPCR-24h) from baseline to Month 9 was numerically lower in patients who developed ADA (41.6%) compared to those who did not (52.7%). The clinical significance of the differences in uPCR reduction according to the presence or absence of ADA is not clear. The presence of ADA did not have a clinically significant effect on the incidence or severity of adverse reactions.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or mutagenicity studies have been conducted with sibeprenlimab-szsi.
Impairment of Fertility
In cynomolgus monkeys intravenously administered sibeprenlimab-szsi at doses of 25, 50, or 100 mg/kg once every two weeks for 26 weeks, no sibeprenlimab-related adverse effects on female menstrual cycle frequency and lengths, male sperm analysis of motility, concentration and count, or male and female reproductive organs were observed at doses up to 100 mg/kg, which provides an approximately 13-fold exposure margin to the clinical exposure at the MRHD based on AUC.
-
14 CLINICAL STUDIES
The effect of VOYXACT on proteinuria was evaluated in a randomized, double-blind, placebo-controlled, multicenter, global study (VISIONARY, NCT05248646) in adults with biopsy-confirmed IgAN, an eGFR ≥30 mL/min/1.73 m2, proteinuria (defined as either urine protein/creatinine ratio based on 24-hour urine collections [uPCR-24h] ≥0.75 g/g or urine protein ≥1.0 g/day), and on a stable and maximally tolerated dose of an angiotensin-converting enzyme inhibitor (ACEi) and/or angiotensin receptor blocker (ARB) with or without a sodium-glucose co-transporter 2 inhibitor (SGLT2i). Patients with other glomerulopathies or those who had been treated with systemic immunosuppressants in the 16 weeks prior to screening were excluded. Patients were randomized 1:1 to receive either VOYXACT or placebo injected subcutaneously once every 4 weeks.
The study enrolled a total of 510 patients. An interim analysis for efficacy was conducted on the first 320 (63%) randomized patients who had the opportunity to reach the Month 9 visit, 152 of whom were randomized to receive VOYXACT while 168 were randomized to receive placebo. Baseline demographics and disease characteristics were generally balanced between treatment groups. At baseline, the median age was 42 years (range 18 to 83 years); 63% were male, 59% were Asian, 38% were White, and 3% were of Other race.
At baseline, mean uPCR-24h was 1.5 g/g, mean estimated glomerular filtration rate (eGFR) was 63 mL/min/1.73 m2, and 74% had hematuria (based on urine dipstick). Approximately 67% of patients had a history of hypertension and 7% had a history of type 2 diabetes mellitus. At baseline, 98% were treated with an ACEi and/or ARB and 40% of patients were also on an SGLT2i.
The primary endpoint was the relative change from baseline in uPCR-24h at Month 9. The mean estimated percent change from baseline in uPCR-24h is shown in Figure 1. The observed geometric mean percent change from baseline over time for uPCR from spot first morning void samples is shown in Figure 2.
Figure 1: Estimated Percent Change Compared to Baseline in uPCR-24h at Month 9 in Patients with IgAN in the VISIONARY Study
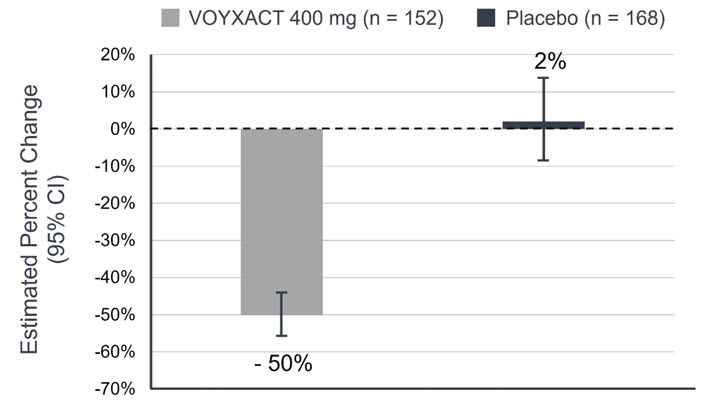
CI = Confidence Interval 24-hour uPCR at Month 9 compared to baseline is defined as uPCR-24h9months/uPCR-24hBaseline. The bar indicates the geometric mean percentage change at 9 months compared with baseline, and the whiskers indicate the 95% CI. Data were included in the analysis regardless of early treatment discontinuation and initiation of confounding therapy (treatment policy strategy). Missing data were imputed using multiple imputation. - * 96.5% CI corresponds to the two-sided significance level of 0.035 for the interim analysis (IA).
VOYXACT -50% Placebo 2% VOYXACT vs. Placebo (96.5% CI)* 51% (43%, 58%) p-value <0.0001 Figure 2: Percent Change Compared to Baseline in Spot uPCR Over Time in Patients with IgAN in the VISIONARY Study
CI = Confidence Interval Spot uPCR at specific week is defined as spot uPCR/spot uPCRbaseline The dots indicate the geometric mean percentage change at specific weeks compared with baseline and the whiskers indicate the corresponding 95% CIs based on observed and non-imputed spot uPCR. 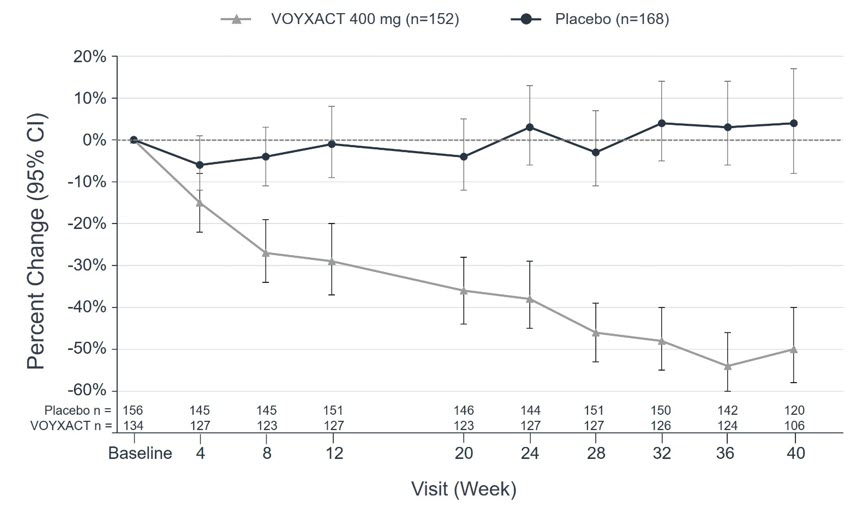
The treatment effect (percentage reduction in uPCR-24h between VOYXACT and placebo) was consistent across the following subgroups and pre-specified stratification factors: sex, age, race, ethnicity, and geographic region, baseline proteinuria (uPCR-24h), baseline eGFR, and SGLT2i use.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
VOYXACT (sibeprenlimab-szsi) injection is supplied as a sterile, preservative-free, clear to opalescent, colorless to yellow solution in a single-dose prefilled syringe with a 27 gauge, ½ inch needle and a needle safety device.
VOYXACT prefilled syringe is not made with natural rubber latex.
VOYXACT 400 mg/2mL (200 mg/mL) prefilled syringe is packaged in an individual carton (NDC: 59148-400-75).
Storage and Handling
Store VOYXACT in a refrigerator at 36°F to 46°F (2°C to 8°C). Keep the prefilled syringe in its original box to protect it from light.
Do not freeze. Do not shake. Do not expose to heat or direct sunlight.
Once VOYXACT prefilled syringe has reached room temperature, do not return it to the refrigerator.
Do not use VOYXACT if it has been at room temperature for 7 days or longer.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Infections
Inform patients that they may be more likely to develop infections when taking VOYXACT. Instruct patients to tell their healthcare provider if they develop signs or symptoms of an infection [see Warnings and Precautions (5.1)].
Hypersensitivity
Inform patients about the signs and symptoms of hypersensitivity reactions. Advise patients to discontinue VOYXACT and seek immediate medical attention for signs or symptoms of hypersensitivity reactions [see Contraindications (4)].
Pregnancy
Advise patients who are exposed to VOYXACT during pregnancy to contact Otsuka Pharmaceutical Development and Commercialization, Inc. at 1-833-869-9228 or www.VOYXACT.com.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
VOYXACT® (VOY-ZAKT)
(sibeprenlimab-szsi)
injection, for subcutaneous useThis Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 11/2025 What is VOYXACT?
VOYXACT is a prescription medicine used to reduce protein in the urine (proteinuria) in adults with a kidney disease called primary immunoglobulin A nephropathy (IgAN) who are at risk for their disease getting worse.
It is not known if VOYXACT is safe and effective in children.Do not take VOYXACT if you: - are allergic to any of the ingredients in VOYXACT. See the end of this Patient Information for a complete list of ingredients in VOYXACT. Stop using VOYXACT and get emergency help if you develop signs and symptoms of an allergic reaction, including:
- breathing problems
- swelling of the face, lips, mouth, tongue or throat
- dizziness or feel lightheaded
- itching
- hives
- skin rash
Before taking VOYXACT, tell your healthcare provider about all of your medical conditions, including if you: - have an infection.
- have received or plan to receive therapy that can lower the ability of your immune system to fight infections (immunosuppressive therapy).
- have recently received or are scheduled to receive an immunization (vaccine). VOYXACT may affect your immune system response to vaccines and increase your risk of infection from live vaccines.
- are pregnant or plan to become pregnant. It is not known if VOYXACT will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if VOYXACT passes into your breast milk or if it will affect your baby. Talk to your healthcare provider about the best way to feed your baby during treatment with VOYXACT.
How should I take VOYXACT? - Read the detailed "Instructions for Use" that comes with VOYXACT for information about how to prepare and inject VOYXACT, and how to properly store and throw away (dispose of) used VOYXACT prefilled syringes.
- Use VOYXACT exactly as your healthcare provider tells you to.
- VOYXACT is given as an injection under the skin (subcutaneous injection).
- Inject VOYXACT under the skin (subcutaneous injection) in the front of your upper legs (thighs) or stomach area (abdomen), avoiding the area 2 inches around the belly button. The back of the upper arms may also be used if a caregiver gives the injection.
- If you miss a dose of VOYXACT, inject the missed dose as soon as possible. Then take your next dose in 4 weeks.
What are the possible side effects of VOYXACT?
VOYXACT can cause serious side effects, including:- immunosuppression and increased risk of infections. VOYXACT weakens your immune system, which may increase your risk of infections. Your healthcare provider will check you for signs and symptoms of infections before you start and during treatment with VOYXACT. Tell your healthcare provider right away if you develop any signs and symptoms of infection during treatment with VOYXACT, including:
- fever
- chills
- feeling tired
- cough
- flu-like symptoms
- aches and pain
- warm, red, or painful skin
The most common side effects of VOYXACT include: upper respiratory tract infection and skin redness at your injection site.
These are not all of the possible side effects of VOYXACT.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store VOYXACT? - Store VOYXACT in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep the VOYXACT prefilled syringe in its original box to protect it from light.
- Let the VOYXACT prefilled syringe come to room temperature up to 77°F (25°C) for 15 to 30 minutes before giving the injection.
- When the VOYXACT prefilled syringe has reached room temperature, do not put it back in the refrigerator.
- Do not use VOYXACT if it has been at room temperature for 7 days or longer.
- Do not freeze the VOYXACT prefilled syringe.
- Do not shake the VOYXACT prefilled syringe.
- Keep the VOYXACT prefilled syringe away from heat and direct sunlight.
- Do not use past the expiration date printed on the box.
General information about the safe and effective use of VOYXACT.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VOYXACT for a condition for which it was not prescribed. Do not give VOYXACT to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about VOYXACT that is written for healthcare professionals.What are the ingredients in VOYXACT?
Active ingredient: sibeprenlimab-szsi
Inactive ingredients: arginine, glutamic acid, histidine, L-histidine hydrochloride monohydrate, polysorbate 80, sorbitol, and Water for Injection.
VOYXACT prefilled syringe is not made with natural rubber latex.
Manufactured by:
Otsuka Pharmaceutical Company, Ltd., Tokyo, 101-8535 Japan
U.S. License No 2387
Distributed by:
Otsuka America Pharmaceutical, Inc.
Rockville, MD 20850, USA
For more information, call 1-833-869-9228 or visit www.VOYXACT.com. -
INSTRUCTIONS FOR USE
VOYXACT® (VOY-ZAKT)
(sibeprenlimab-szsi)
injection, for subcutaneous use
Single-Dose Prefilled SyringeThis Instructions for Use contains information on how to inject VOYXACT. Read and follow this Instructions for Use before you inject VOYXACT prefilled syringe for the first time and each time you get a refill. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. Your healthcare provider should show you or your caregiver how to prepare and inject VOYXACT the right way before you use or give it for the first time.
Important Information You Need to Know Before Injecting VOYXACT Prefilled Syringe
- VOYXACT prefilled syringe is for injection under the skin (subcutaneous injection) use only.
- Do not remove the grey needle cap from the prefilled syringe until you are ready to inject.
- Each prefilled syringe has a needle guard. It will automatically cover the needle after the injection is completed.
- Do not pull back on the plunger at any time.
- Do not use if the VOYXACT prefilled syringe has been damaged.
- Do not use if the VOYXACT prefilled syringe has been dropped after removing the needle cap.
Storing VOYXACT Prefilled Syringe
- Store your VOYXACT prefilled syringe in a refrigerator between 36°F to 46°F (2°C to 8°C). Keep the prefilled syringe in its original box to protect it from light.
- Let the VOYXACT prefilled syringe come to room temperature up to 77°F (25°C) for 15 to 30 minutes before giving an injection.
- When the VOYXACT prefilled syringe has reached room temperature, do not put it back in the refrigerator.
- Do not use the VOYXACT prefilled syringe if it has been at room temperature for 7 days or longer.
- Do not freeze the VOYXACT prefilled syringe.
- Do not shake the VOYXACT prefilled syringe.
- Keep the VOYXACT prefilled syringe away from heat and direct sunlight.
- Throw away (dispose of) any VOYXACT prefilled syringe if it is expired. See Step 11 for instructions on how to throw away (dispose of) the VOYXACT prefilled syringe.
- Keep VOYXACT prefilled syringe and all medicines out of the reach of children.
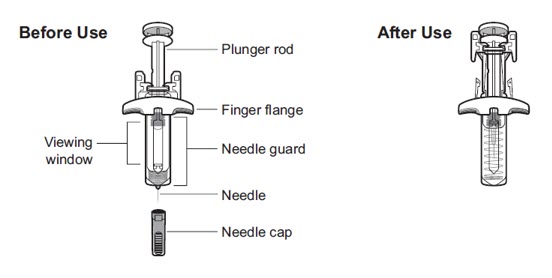
Your VOYXACT Single-Dose Prefilled Syringe
Getting Your Supplies Ready
Step 1: Remove the VOYXACT prefilled syringe box from the refrigerator
Take the box containing VOYXACT prefilled syringe out of the refrigerator and check the expiration date (EXP) on the side of the box.
- Do not use the prefilled syringe if the seals on the box have been broken.
- Do not use the prefilled syringe if the expiration date (EXP) printed on the box has passed.
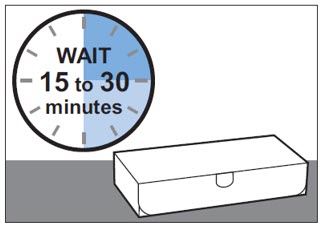
Step 2: Wait 15 to 30 minutes before use
Wait 15 to 30 minutes to let the prefilled syringe come to room temperature naturally (see Figure A).
- Do not warm the prefilled syringe any other way.
- Do not let the prefilled syringe sit in direct sunlight.
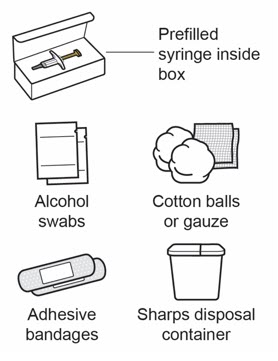
Step 3: Gather supplies
Gather supplies that are not included in the VOYXACT prefilled syringe box:
- Alcohol swabs
- Cotton balls or gauze
- Adhesive bandages
- Sharps disposal container (see Step 11)
Preparing to Inject the VOYXACT Prefilled Syringe
Step 4: Take the VOYXACT prefilled syringe out of the box
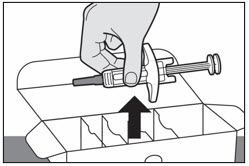
Hold the middle of the prefilled syringe (near the viewing window) to carefully lift the prefilled syringe out of the box (see Figure B).
- Do not lift the prefilled syringe by the plunger.
- Do not shake or roll the prefilled syringe.
- Do not remove the grey needle cap from the prefilled syringe until Step 8 and you are ready to inject.
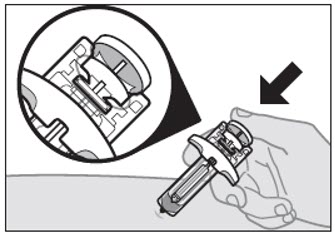
Step 5: Inspect the VOYXACT Prefilled Syringe
- Make sure that VOYXACT appears on the labels.
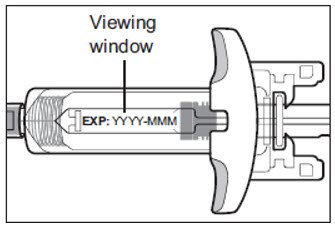
- Check the expiration date (EXP) printed on the prefilled syringe (see Figure C).
- Check the medicine in the prefilled syringe through the viewing window. It is normal to see air bubbles. The medicine inside should be clear to slightly pearly and colorless to yellow (see Figure C).
-
Do not use VOYXACT prefilled syringe if:
- - the expiration date printed on the prefilled syringe label has passed.
- - the medicine is cloudy, discolored, or has particles floating in it.
- - any part of the prefilled syringe is damaged, broken, or the needle guard has been activated.
- Do not try to remove air bubble(s) from the prefilled syringe.
Step 6: Choose the injection site
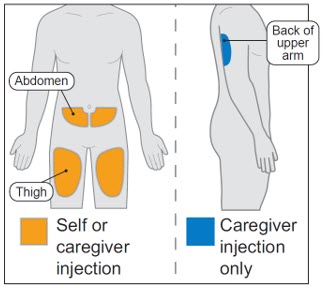
Choose an injection site on your bare skin from 1 of the following areas (see Figure D):
- front of upper thigh
- stomach area (abdomen), avoiding the area 2 inches around the belly button.
For caregiver only: back of the upper arm may also be used. - Do not inject into the same spot you used for your last injection.
- Do not inject into moles, scars, or bruises or skin that is tender, damaged, hard or red.
Step 7: Clean the injection site
Wash your hands with soap and clean water (see Figure E).
- Clean the injection site with an alcohol swab and let the skin dry (see Figure F).
- Do not touch the area again before injecting.
- Do not fan or blow on the injection site after you have cleaned it.
Injecting VOYXACT
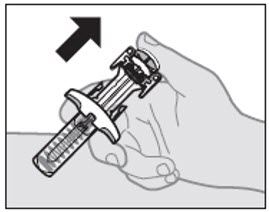
Step 8: Remove the needle cap
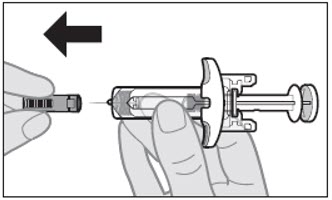
When you are ready to inject, hold the prefilled syringe with 1 hand, pull the needle cap straight off with the other hand (see Figure G).
- You may see a drop of liquid at the end of the needle. This is normal.
- Do not touch or recap the needle or let it touch any surface.
- Do not touch or pull back the plunger.
- Do not use a prefilled syringe with a bent or broken needle.
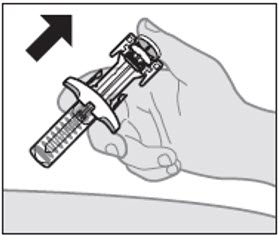
Step 9: Pinch the skin and insert needle
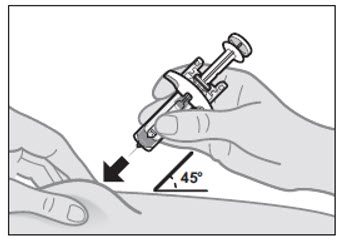
With the needle cap off, hold the prefilled syringe in 1 hand. With your other hand, gently pinch the skin at the injection site (see Figure H).
- It is important to pinch enough skin to inject under the skin and not into the muscle.
Insert the entire needle at a 45-degree angle into the pinched skin using a dart-like motion (see Figure H).
- Avoid touching the plunger until the needle is inserted.
Step 10: Complete the Injection
Push the plunger as far as it will go and it stops, and all the medicine is injected (see Figure I).
Take your thumb off the plunger to allow the needle guard to automatically cover the exposed needle (see Figure J).
Gently lift the syringe away from the injection site (see Figure K).
- There may be a small amount of blood or liquid at the injection site. Hold pressure over your skin with a cotton ball or gauze until any bleeding stops.
- Apply an adhesive bandage, if needed.
- Do not rub or massage the injection site.
Disposing of the VOYXACT Prefilled Syringe
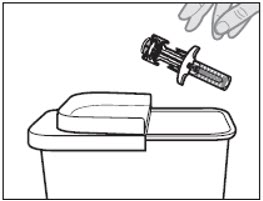
Step 11: Throw away (dispose of) the VOYXACT prefilled syringe
Throw away (dispose of) the used prefilled syringe in a FDA-cleared sharps disposal container right away after use (see Figure L). Do not recycle or throw away the prefilled syringe in your household trash.
- Throw away the remaining supplies in your household trash or sharps container.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- - made of a heavy-duty plastic,
- - can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- - upright and stable during use,
- - leak-resistant, and
- - properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be local or state laws about how to throw away needles and syringes.
- For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not recycle your used sharps disposal container.
If you have questions or concerns about your VOYXACT prefilled syringe, please call your healthcare provider. You can also call 1-800-441-6763 or visit www.VOYXACT.com for more information.
Manufactured by:
Otsuka Pharmaceutical Company, Ltd.Tokyo 101-8535 Japan
U.S. License No 2387Distributed by:
Otsuka America Pharmaceutical, Inc.
Rockville, MD 20850 USAThis Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: Nov 2025 -
PRINCIPAL DISPLAY PANEL - 200 mg/mL Syringe Carton
NDC: 59148-400-75
Rx only
Voyxact®
(sibeprenlimab-szsi)
Injection400 mg/2 mL
(200 mg/mL)For Subcutaneous Use Only
Contains one single-dose prefilled syringe.
Discard after use.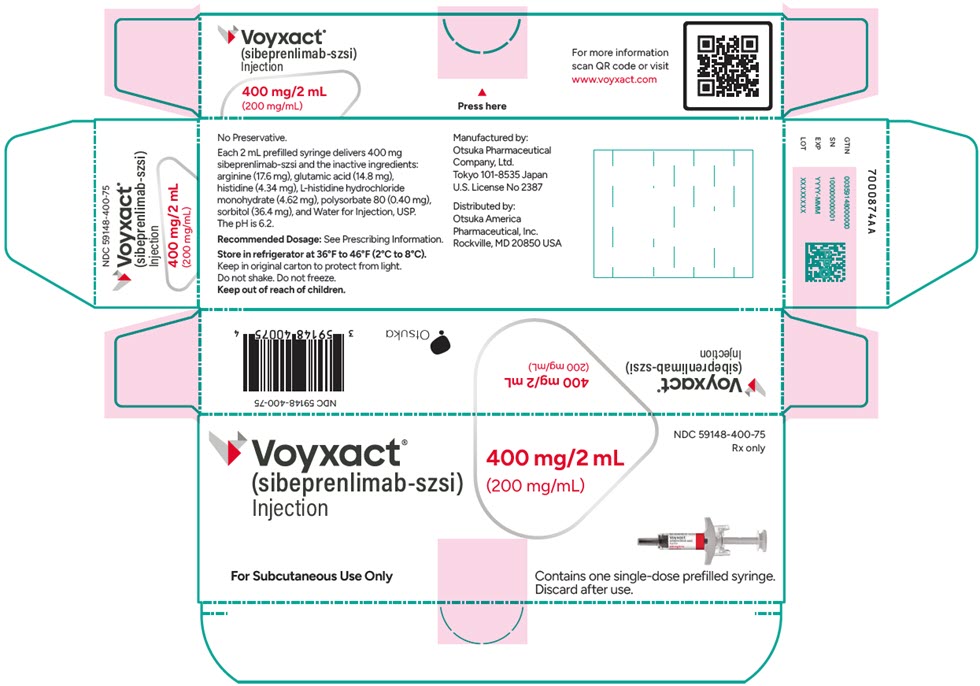
-
INGREDIENTS AND APPEARANCE
VOYXACT
sibeprenlimab injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59148-400 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sibeprenlimab (UNII: GHX28QZ7DD) (sibeprenlimab - UNII:GHX28QZ7DD) sibeprenlimab 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength arginine (UNII: 94ZLA3W45F) glutamic acid (UNII: 3KX376GY7L) histidine (UNII: 4QD397987E) polysorbate 80 (UNII: 6OZP39ZG8H) sorbitol (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (clear to opalescent, colorless to yellow solution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59148-400-75 1 in 1 CARTON 11/25/2025 1 2 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761434 11/25/2025 Labeler - Otsuka America Pharmaceutical, Inc. (008314390)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.