ANDALOU BB UNTINTED WITH SPF-30- zinc oxide lotion
Andalou BB Untinted with SPF-30 by
Drug Labeling and Warnings
Andalou BB Untinted with SPF-30 by is a Otc medication manufactured, distributed, or labeled by Andalou naturals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Daily, alone or under make-up.
- Apply liberally 15 minutes before sun exposure and as needed.
- Reapply at least every 2 hours.
- Use water resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10am-2pm, wear long sleeve shirts, pants and sunglasses.
- Children under 6 months: ask a doctor.
-
Inactive Ingredients
Aloe Barbadensis Juice1, Simmondsia Chinensis (Jojoba) and Helianthus Annuus (Sunflower) Oils1, Sorbitan Stearate, Glyceryl Stearate, Fruit Stem Cell and BioActive 8 Berry Complex1, Caprylic/Capric Triglycerides, Hyaluronic Acid, Salvia Officinalis (Sage), Serenoa Rapens (Saw Palmetto) and Salix Alba (Willow Bark) Extracts1, Punica Granatum (Pomegranate) Oil1, Magnesium Ascorbyl Phosphate (Vitamin C), Algae Extract, Zinc Gluconate, Magnesium Sulfate, Aspalathus Linearis (Rooibos), Hibiscus Rosa-Sinensis and Camellia Sinensis (White Tea) Extracts12, Phenethyl Alcohol, Ethylhexylglycerin, Citrus Tangerina (Tangerine) and Cymbopogon Martini (Palmarosa) Oils1
- 1 Organic
- 2 Fair Trade
- Other Information
- Questions
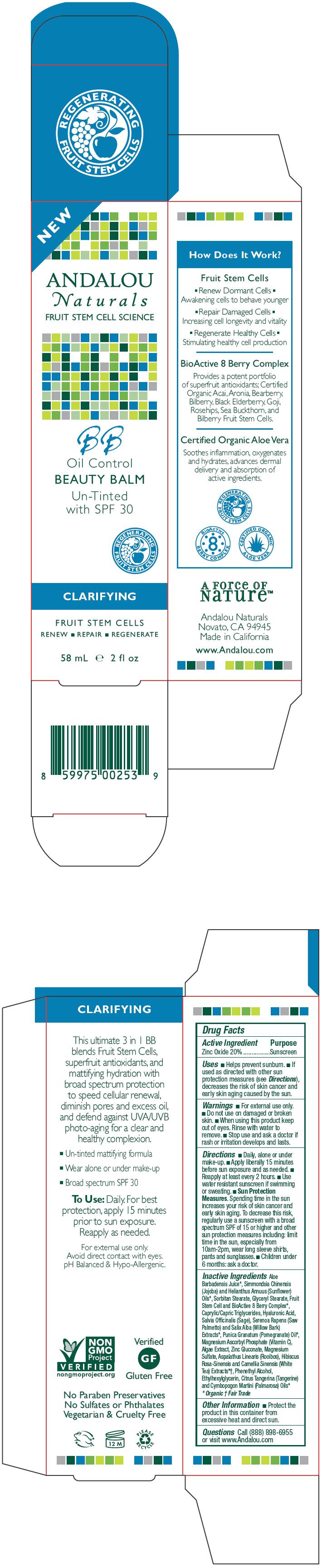
- PRINCIPAL DISPLAY PANEL - 58 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
ANDALOU BB UNTINTED WITH SPF-30
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55560-0101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 20 g in 100 mL Inactive Ingredients Ingredient Name Strength glyceryl monostearate (UNII: 230OU9XXE4) sage (UNII: 065C5D077J) saw palmetto (UNII: J7WWH9M8QS) willow bark (UNII: S883J9JDYX) pomegranate seed oil (UNII: 0UI45XV0T6) aloe vera leaf (UNII: ZY81Z83H0X) zinc gluconate (UNII: U6WSN5SQ1Z) medium-chain triglycerides (UNII: C9H2L21V7U) sorbitan monostearate (UNII: NVZ4I0H58X) mandarin oil (UNII: NJO720F72R) magnesium sulfate (UNII: DE08037SAB) helianthus annuus flowering top (UNII: BKJ0J3D1BP) jojoba oil (UNII: 724GKU717M) tocopherol (UNII: R0ZB2556P8) magnesium ascorbyl phosphate (UNII: 0R822556M5) hyaluronate sodium (UNII: YSE9PPT4TH) palmarosa oil (UNII: 0J3G3O53ST) phenylethyl alcohol (UNII: ML9LGA7468) ethylhexylglycerin (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55560-0101-1 58 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/10/2012 Labeler - Andalou naturals (011472720)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.