FEMALE ENERGY- chaste tree, arnica montana, caulophyllum thalictroides root, cinchona officinalis bark, turnera diffusa leafy twig, herring sperm dna, lactuca virosa, onosmodium virginianum whole, sus scrofa ovary, phosphoric acid, sus scrofa pituitary gland, sepia officinalis juice, thuja occidentalis leafy twig spray
Female Energy by
Drug Labeling and Warnings
Female Energy by is a Homeopathic medication manufactured, distributed, or labeled by Sunway Biotech LLC, Integra Health International, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
Active Ingredients: equal volumes of each ingredient:
agnus castus 3x HPUS, arnica montana 3X HPUS, caulophyllum thalictroides 6X HPUS, cinchona officinalis 3X HPUS, damiana 1X HPUS, DNA 6X HPUS, lactuca virosa 3X HPUS, onosmodium virginianum 30C HPUS, oophorinum 30C HPUS, phosphoricum acidum 200C HPUS, pituitarium posterium 12X HPUS, sepia 30C HPUS, thuja occidentalis 30C HPUS.
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
WARNINGS
If pregnant or breast feeding, ask a doctor before using product. Stop use and ask a doctor if symptoms persist more than 3 days, worsen, or if new symptoms occur.
Tamper Evident Seal: Do not use if seal around neck of bottle is broken or missing. The letter HPUS indicate that ingredients are officially included in the Homeopathic Pharmacopoeia of the United States.
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FEMALE ENERGY
chaste tree, arnica montana, caulophyllum thalictroides root, cinchona officinalis bark, turnera diffusa leafy twig, herring sperm dna, lactuca virosa, onosmodium virginianum whole, sus scrofa ovary, phosphoric acid, sus scrofa pituitary gland, sepia officinalis juice, thuja occidentalis leafy twig sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 27281-009 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHASTE TREE (UNII: 433OSF3U8A) (CHASTE TREE - UNII:433OSF3U8A) CHASTE TREE 1.5 [hp_C] in 1 mg ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1.5 [hp_C] in 1 mg CAULOPHYLLUM THALICTROIDES ROOT (UNII: JTJ6HH6YEH) (CAULOPHYLLUM THALICTROIDES ROOT - UNII:JTJ6HH6YEH) CAULOPHYLLUM THALICTROIDES ROOT 3 [hp_C] in 1 mg CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 1.5 [hp_C] in 1 mg TURNERA DIFFUSA LEAFY TWIG (UNII: RQ2CFA7WWJ) (TURNERA DIFFUSA LEAFY TWIG - UNII:RQ2CFA7WWJ) TURNERA DIFFUSA LEAFY TWIG 0.5 [hp_C] in 1 mg HERRING SPERM DNA (UNII: 51FI676N6F) (HERRING SPERM DNA - UNII:51FI676N6F) HERRING SPERM DNA 3 [hp_C] in 1 mg LACTUCA VIROSA (UNII: 6D74QW4H67) (LACTUCA VIROSA - UNII:6D74QW4H67) LACTUCA VIROSA 1.5 [hp_C] in 1 mg ONOSMODIUM VIRGINIANUM WHOLE (UNII: 604NK4250S) (ONOSMODIUM VIRGINIANUM WHOLE - UNII:604NK4250S) ONOSMODIUM VIRGINIANUM WHOLE 30 [hp_C] in 1 mg SUS SCROFA OVARY (UNII: S7YTV04R8O) (SUS SCROFA OVARY - UNII:S7YTV04R8O) SUS SCROFA OVARY 30 [hp_C] in 1 mg PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 200 [hp_C] in 1 mg SUS SCROFA PITUITARY GLAND (UNII: E8S87O660T) (SUS SCROFA PITUITARY GLAND - UNII:E8S87O660T) SUS SCROFA PITUITARY GLAND 6 [hp_C] in 1 mg SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 1 mg THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 30 [hp_C] in 1 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 27281-009-96 30 mg in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/07/2014 Labeler - Sunway Biotech LLC (019560802) Establishment Name Address ID/FEI Business Operations Integra Health International, S.A. de C.V. 589880301 manufacture(27281-009)
Trademark Results [Female Energy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
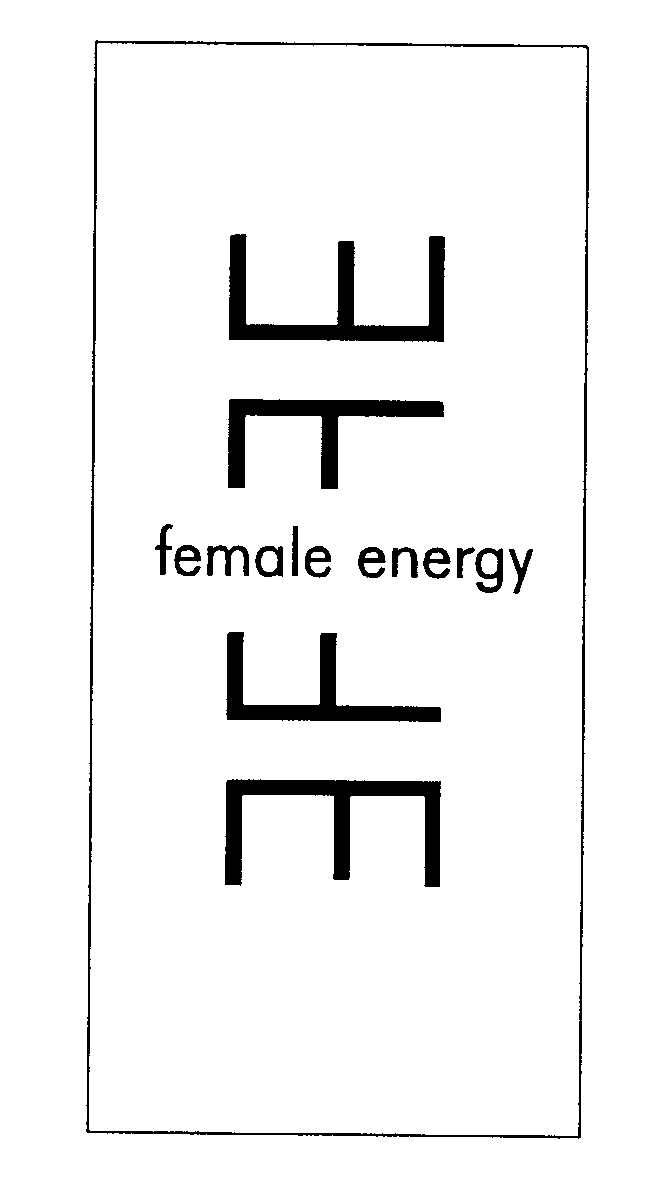 FEMALE ENERGY 78138378 not registered Dead/Abandoned |
Louthain, P. Kim 2002-06-24 |
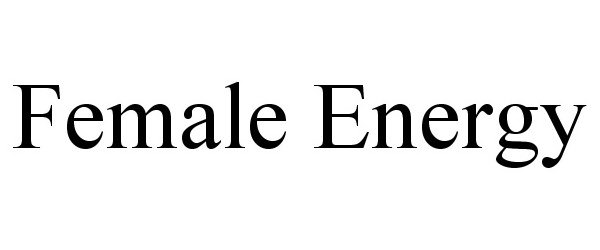 FEMALE ENERGY 77397797 not registered Dead/Abandoned |
Century Systems Inc. 2008-02-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
