STAYKLEAR- alcohol liquid
STAYKLEAR by
Drug Labeling and Warnings
STAYKLEAR by is a Otc medication manufactured, distributed, or labeled by eHealth Nexus LLC, Nutrix. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
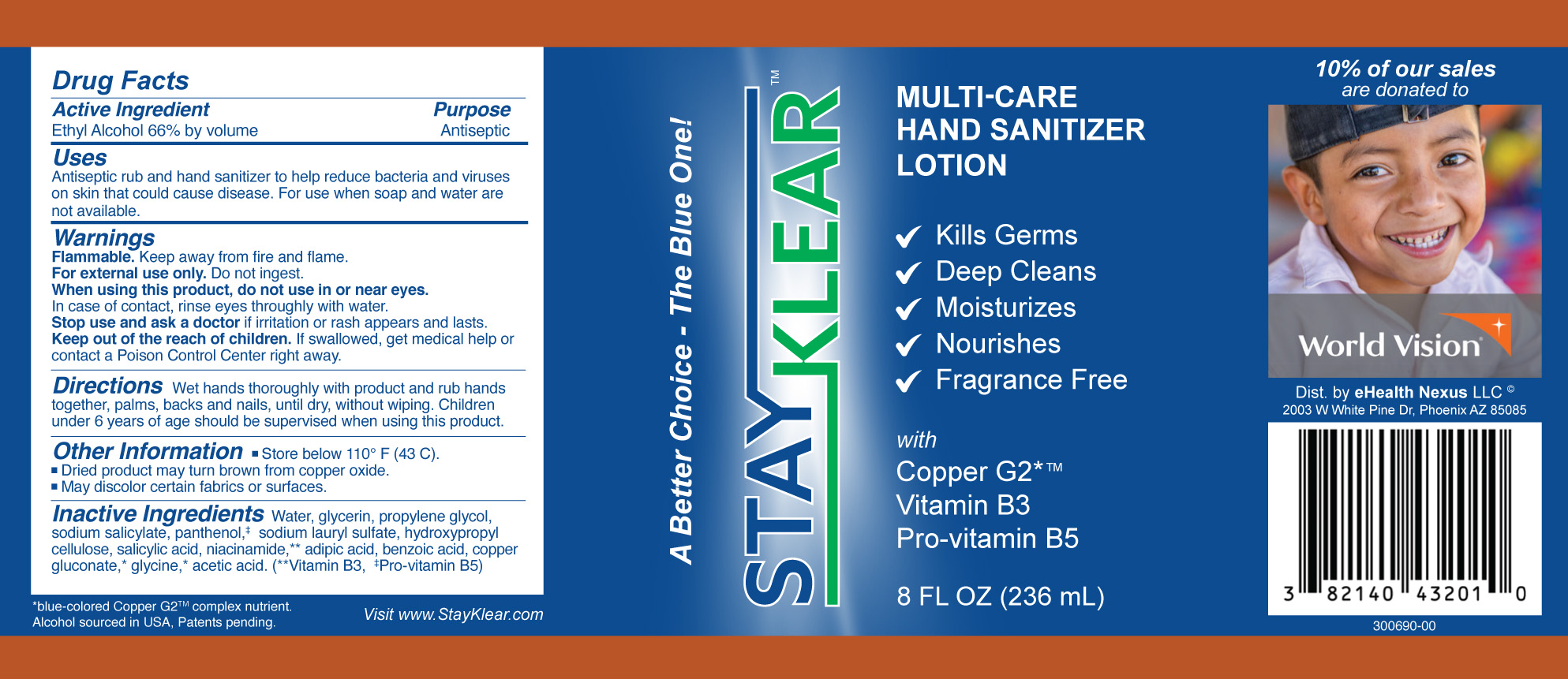
- Active Ingredient
- Purpose
- Uses
- Warnings:
- KEEP OUT OF REACH OF CHILDREN
- Directions:
- Other Information:
-
Inactive Ingredients:
Water, glycerin, propylene glycol, sodium salicylate, panthenol,‡ sodium lauryl sulfate, salicylic acid, hydroxypropyl cellulose, lactic acid, niacinamide,** isopropyl myristate, fragrance, copper gluconate, benzoic acid, orange oil, adipic acid, acetic acid, glycine, sodium chloride. **Vitamin B3, ‡Pro-vitamin B5
- Package Label
-
INGREDIENTS AND APPEARANCE
STAYKLEAR
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82140-432 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.6 kg in 1 kg Inactive Ingredients Ingredient Name Strength VANILLIN (UNII: CHI530446X) 0.001 kg in 1 kg BENZYL BENZOATE (UNII: N863NB338G) 0.001 kg in 1 kg ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) 0.003 kg in 1 kg LACTIC ACID (UNII: 33X04XA5AT) 0.003 kg in 1 kg WATER (UNII: 059QF0KO0R) 0.29 kg in 1 kg HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) 0.0055 kg in 1 kg PANTHENOL (UNII: WV9CM0O67Z) 0.008 kg in 1 kg PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 0.016 kg in 1 kg SODIUM SALICYLATE (UNII: WIQ1H85SYP) 0.008 kg in 1 kg GLYCERIN (UNII: PDC6A3C0OX) 0.034 kg in 1 kg SODIUM LAURYL SULFATE (UNII: 368GB5141J) 0.006 kg in 1 kg NIACINAMIDE (UNII: 25X51I8RD4) 0.0032 kg in 1 kg ADIPIC ACID (UNII: 76A0JE0FKJ) 0.0015 kg in 1 kg BENZOIC ACID (UNII: 8SKN0B0MIM) 0.002 kg in 1 kg SALICYLIC ACID (UNII: O414PZ4LPZ) 0.006 kg in 1 kg GLYCINE (UNII: TE7660XO1C) 0.001 kg in 1 kg COPPER GLUCONATE (UNII: RV823G6G67) 0.0022 kg in 1 kg ACETIC ACID (UNII: Q40Q9N063P) 0.0013 kg in 1 kg SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.0006 kg in 1 kg ORANGE OIL (UNII: AKN3KSD11B) 0.0015 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82140-432-01 0.213 kg in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 09/03/2021 2 NDC: 82140-432-02 0.053 kg in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 09/16/2021 3 NDC: 82140-432-03 0.09 kg in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/05/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/16/2021 Labeler - eHealth Nexus LLC (067923713) Registrant - eHealth Nexus LLC (067923713) Establishment Name Address ID/FEI Business Operations Nutrix 117341868 manufacture(82140-432)
Trademark Results [STAYKLEAR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STAYKLEAR 90316732 not registered Live/Pending |
eHealth Nexus LLC 2020-11-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.