BACKPRIN- magnesium salicylate tetrahydrate tablet, film coated
BACKPRIN by
Drug Labeling and Warnings
BACKPRIN by is a Otc medication manufactured, distributed, or labeled by HART Health. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Reye's syndrome:
Children and teenagers should not use this medicine for chicken pox or flu symptoms before a doctor is consulted about Reye's Syndrome, a rare but serious illness reported to be associated with salicylates.
Allergy alert:.
Magnesium salicylate may cause a severe allergic reaction which may include:
■ hives
■ facial swelling
■ asthma (wheezing)
■ shock
Stomach bleeding warning:
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
■ are age 60 or older
■ have had stomach ulcers or bleeding problems
■ take a blood thinning (anticoagulant) or steroid drug
■ take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
■ have 3 or more alcoholic drinks every day while using this product
■ take more or for a longer time than directed
- DO NOT USE
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
-
STOP USE
Stop use and ask a doctor if
■ an allergic reaction occurs, seek medical help right away
■ fever worsens or lasts more than 3 days
■ pain worsens or lasts more than 10 days
■ you experience any of the following signs of stomach bleeding: feel faint, vomit blood, have bloody or black stools, or stomach pain that does not get better
■ ringing in the ears or loss of hearing occurs
■ new symptoms occur
■ redness or swelling is present
These could be signs of a serious condition.
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
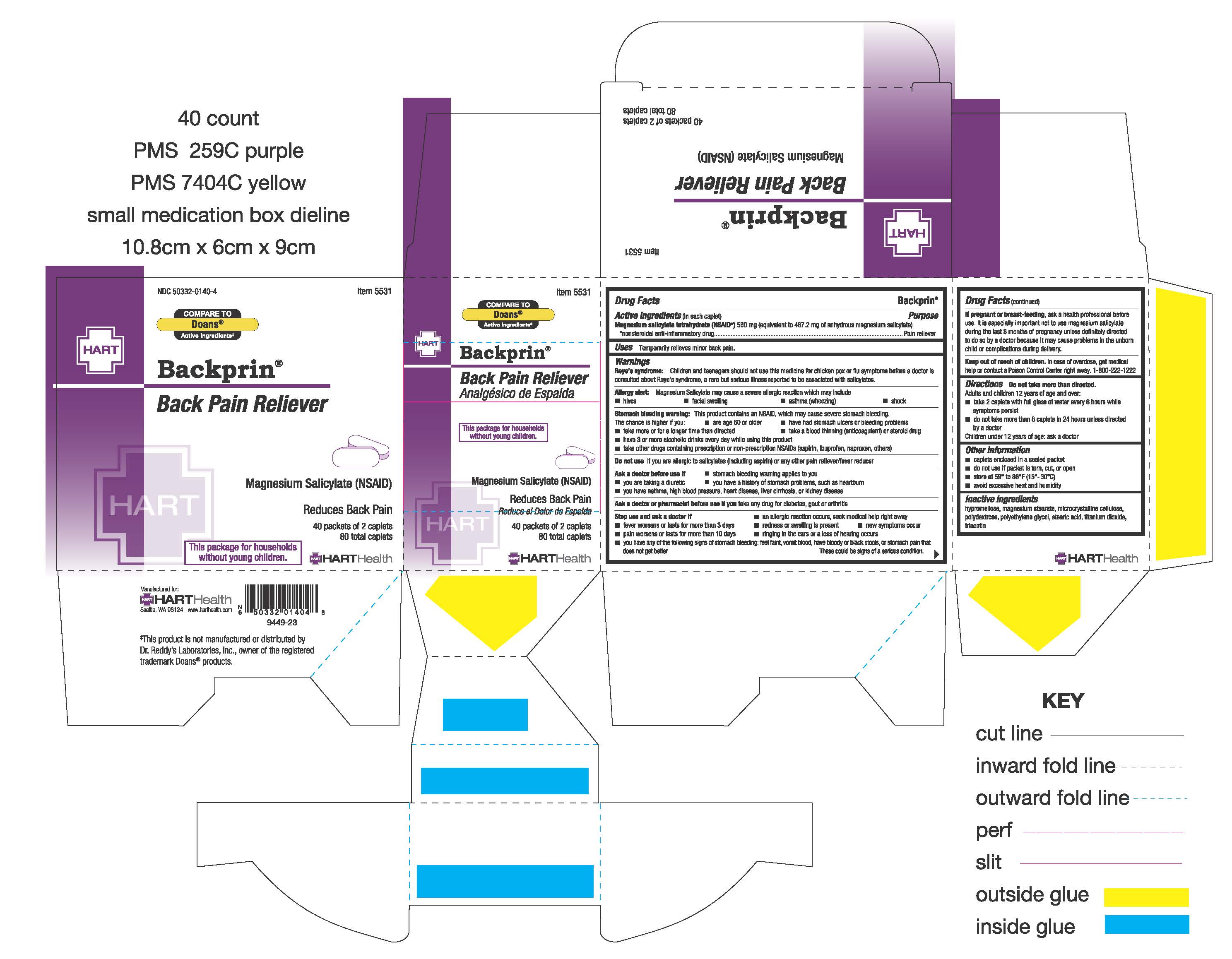
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BACKPRIN
magnesium salicylate tetrahydrate tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50332-0140 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM SALICYLATE (UNII: 41728CY7UX) (SALICYLIC ACID - UNII:O414PZ4LPZ) MAGNESIUM SALICYLATE 580 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYDEXTROSE (UNII: VH2XOU12IE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white Score no score Shape CAPSULE (Caplet) Size 17mm Flavor Imprint Code 44;338 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50332-0140-4 40 in 1 BOX 01/09/2023 1 2 in 1 PACKET; Type 0: Not a Combination Product 2 NDC: 50332-0140-7 100 in 1 BOX 01/09/2023 2 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 01/09/2023 Labeler - HART Health (069560969)
Trademark Results [BACKPRIN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BACKPRIN 76244726 2612229 Live/Registered |
NorMed, Inc. 2001-04-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.