HUMALOG- insulin lispro injection, solution HUMALOG KWIKPEN- insulin lispro injection, solution HUMALOG JUNIOR KWIKPEN- insulin lispro injection, solution HUMALOG TEMPO PEN- insulin lispro injection, solution
Humalog by
Drug Labeling and Warnings
Humalog by is a Prescription medication manufactured, distributed, or labeled by Eli Lilly and Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HUMALOG safely and effectively. See full prescribing information for HUMALOG.
HUMALOG (insulin lispro injection), for subcutaneous or intravenous use
Initial U.S. Approval: 1996INDICATIONS AND USAGE
HUMALOG is a rapid acting human insulin analog indicated to improve glycemic control in adults and children with diabetes mellitus. (1)
DOSAGE AND ADMINISTRATION
- See Full Prescribing Information for important administration instructions. (2.1, 2.2, 2.3, 2.4)
- Subcutaneous injection (2.2):
- Administer HUMALOG® U-100 or U-200 by subcutaneous injection into the abdominal wall, thigh, upper arm, or buttocks within 15 minutes before a meal or immediately after a meal.
- Rotate injection sites to reduce risk of lipodystrophy and localized cutaneous amyloidosis.
- Continuous subcutaneous infusion (Insulin Pump) (2.2):
- Administer HUMALOG U-100 by continuous subcutaneous infusion using an insulin pump in a region recommended in the instructions from the pump manufacturer.
- Rotate infusion sites to reduce risk of lipodystrophy and localized cutaneous amyloidosis.
- DO NOT administer HUMALOG U-200 by continuous subcutaneous infusion.
- Intravenous Infusion (2.2):
- Administer HUMALOG U-100 by intravenous infusion ONLY after dilution and under medical supervision. DO NOT administer HUMALOG U-200 by intravenous infusion.
- The dosage of HUMALOG must be individualized based on the route of administration and the individual's metabolic needs, blood glucose monitoring results and glycemic control goal. (2.3)
- Do not perform dose conversion when using the HUMALOG U-100 or U-200 prefilled pens. The dose window shows the number of insulin units to be delivered and no conversion is needed. (2.1, 2.3)
- Do not mix HUMALOG U-200 with any other insulin. (2.4)
DOSAGE FORMS AND STRENGTHS
Injection: 100 units/mL (U-100) is available as: (3)
- 10 mL multiple-dose vial
- 3 mL multiple-dose vial
- 3 mL single-patient-use Humalog KwikPen®
- 3 mL single-patient-use Humalog Tempo Pen™
- 3 mL single-patient-use Humalog® Junior KwikPen®
- 3 mL single-patient-use cartridges
Injection: 200 units/mL (U-200) is available as: (3)
- 3 mL single-patient-use Humalog KwikPen®
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Never share a HUMALOG prefilled pen, cartridge, reusable pen compatible with Lilly 3 mL cartridges, or syringe between patients, even if the needle is changed. (5.1)
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Make changes to a patient's insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) under close medical supervision with increased frequency of blood glucose monitoring. (5.2)
- Hypoglycemia: May be life-threatening. Monitor blood glucose and increase monitoring frequency with changes to insulin dosage, use of glucose lowering medications, meal pattern, physical activity; in patients with renal or hepatic impairment; and in patients with hypoglycemia unawareness. (5.3, 7, 8.6, 8.7)
- Hypoglycemia Due to Medication Errors: Accidental mix-ups between insulin products can occur. Instruct patients to check insulin labels before injection. Do not transfer HUMALOG U-200 from the HUMALOG KwikPen to a syringe as overdosage and severe hypoglycemia can result. (5.4)
- Hypersensitivity Reactions: May be life-threatening. Discontinue HUMALOG, monitor and treat if indicated. (5.5)
- Hypokalemia: May be life-threatening. Monitor potassium levels in patients at risk of hypokalemia and treat if indicated. (5.6)
- Fluid Retention and Heart Failure with Concomitant Use of Thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; consider dosage reduction or discontinuation if heart failure occurs. (5.7)
- Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction: Monitor glucose and administer HUMALOG U-100 by subcutaneous injection if pump malfunction occurs. (5.8)
ADVERSE REACTIONS
Adverse reactions associated with HUMALOG include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Eli Lilly and Company at 1-800-LillyRx (1-800-545-5979) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Pediatrics: Not studied in children with type 2 diabetes or in children with type 1 diabetes <3 years of age. (8.4)
See 17 for PATIENT COUNSELING INFORMATION, FDA-approved patient labeling and FDA-approved patient labeling.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Route of Administration
2.3 Dosage Information
2.4 Dosage Adjustment Due to Drug Interactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Never Share a HUMALOG Prefilled Pen, Cartridge, Reusable Pen Compatible with Lilly 3 mL Cartridges1, or Syringe Between Patients
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
5.3 Hypoglycemia
5.4 Hypoglycemia Due to Medication Errors
5.5 Hypersensitivity Reactions
5.6 Hypokalemia
5.7 Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists
5.8 Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs That May Increase the Risk of Hypoglycemia
7.2 Drugs That May Decrease the Blood Glucose Lowering Effect of HUMALOG
7.3 Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of HUMALOG
7.4 Drugs That May Blunt Signs and Symptoms of Hypoglycemia
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Type 1 Diabetes – Adults and Adolescents
14.2 Type 2 Diabetes – Adults
14.3 Type 1 Diabetes – Pediatric and Adolescents
14.4 Type 1 Diabetes – Adults Continuous Subcutaneous Insulin Infusion
14.5 Type 1 Diabetes – Pediatric Continuous Subcutaneous Insulin Infusion
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
16.3 Preparation and Handling
16.4 Admixture for Intravenous Administration
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Always check insulin labels before administration [see Warnings and Precautions (5.4)].
- Inspect HUMALOG visually before use. It should appear clear and colorless. Do not use HUMALOG if particulate matter or coloration is seen.
- Use HUMALOG prefilled pens with caution in patients with visual impairment that may rely on audible clicks to dial their dose.
- Do NOT mix HUMALOG U-100 with other insulins when administering using a continuous subcutaneous infusion pump.
- Do NOT transfer HUMALOG U-200 from the prefilled pen to a syringe for administration [see Warnings and Precautions (5.4)].
- Do NOT perform dose conversion when using any HUMALOG U-100 or U-200 prefilled pens. The dose window shows the number of insulin units to be delivered and no conversion is needed.
- Do NOT mix HUMALOG U-200 with any other insulins.
- Do NOT administer HUMALOG U-200 using a continuous subcutaneous infusion pump (i.e., insulin pump).
- Do NOT administer HUMALOG U-200 intravenously.
2.2 Route of Administration
Subcutaneous Injection: HUMALOG U-100 or U-200
- Administer the dose of HUMALOG U-100 or HUMALOG U-200 within fifteen minutes before a meal or immediately after a meal by injection into the subcutaneous tissue of the abdominal wall, thigh, upper arm, or buttocks. To reduce the risk of lipodystrophy and localized cutaneous amyloidosis, rotate the injection site within the same region from one injection to the next. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2) and Adverse Reactions (6)].
- During changes to a patient's insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2)].
- HUMALOG administered by subcutaneous injection should generally be used in regimens with an intermediate- or long-acting insulin.
- The HUMALOG U-100 KwikPen, HUMALOG U-100 Tempo Pen and HUMALOG U-200 KwikPen each dial in 1 unit increments and delivers a maximum dose of 60 units per injection.
- The HUMALOG U-100 Junior KwikPen dials in 0.5 unit increments and delivers a maximum does of 30 units per injection.
Continuous Subcutaneous Infusion (Insulin Pump): HUMALOG U-100 ONLY
- Do NOT administer HUMALOG U-200 using a continuous subcutaneous infusion pump.
- Administer HUMALOG U-100 by continuous subcutaneous infusion in a region recommended in the instructions from the pump manufacturer. Rotate infusion sites within the same region to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2) and Adverse Reactions (6)].
- During changes to a patient's insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2)].
- Follow healthcare professional recommendations when setting basal and meal time infusion rate.
- Do NOT dilute or mix HUMALOG U-100 when administering by continuous subcutaneous infusion.
- Change HUMALOG U-100 in the pump reservoir at least every 7 days.
- Change the infusion sets and the infusion set insertion site at least every 3 days.
- Do NOT expose HUMALOG U-100 in the pump reservoir to temperatures greater than 98.6°F (37°C).
- Use HUMALOG U-100 in pump systems suitable for insulin infusion [see Patient Counseling Information (17)].
Intravenous Administration: HUMALOG U-100 ONLY
- Do NOT administer HUMALOG U-200 intravenously.
- Dilute HUMALOG U-100 to concentrations from 0.1 unit/mL to 1.0 unit/mL using 0.9% Sodium Chloride Injection, USP.
- Administer HUMALOG U-100 intravenously ONLY under medical supervision with close monitoring of blood glucose and potassium levels to avoid hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.6) and How Supplied/Storage and Handling (16.4)].
2.3 Dosage Information
- Individualize and adjust the dosage of HUMALOG based on route of administration, the individual's metabolic needs, blood glucose monitoring results and glycemic control goal.
- Dosage adjustments may be needed with changes in physical activity, changes in meal patterns (i.e., macronutrient content or timing of food intake), changes in renal or hepatic function or during acute illness [see Warnings and Precautions (5.2, 5.3) and Use in Specific Populations (8.6, 8.7)].
- Do NOT perform dose conversion when using any HUMALOG U-100 or U-200 prefilled pens. The dose window shows the number of insulin units to be delivered and no conversion is needed.
2.4 Dosage Adjustment Due to Drug Interactions
- Dosage adjustment may be needed when HUMALOG is coadministered with certain drugs [see Drug Interactions (7)].
- Dosage adjustment may be needed when switching from another insulin to HUMALOG [see Warnings and Precautions (5.2)].
- Instructions for Mixing with Other Insulins
HUMALOG U-100 subcutaneous injection route - HUMALOG U-100 may be mixed with NPH insulin preparations ONLY.
- If HUMALOG U-100 is mixed with NPH insulin, HUMALOG U-100 should be drawn into the syringe first. Injection should occur immediately after mixing.
HUMALOG U-100 continuous subcutaneous infusion route (Insulin Pump) - Do NOT mix HUMALOG U-100 with any other insulin.
HUMALOG U-200 subcutaneous injection route - Do NOT mix with any other insulin.
-
3 DOSAGE FORMS AND STRENGTHS
Injection: 100 units per mL (U-100) clear and colorless solution available as:
- 10 mL multiple-dose vial
- 3 mL multiple-dose vial
- 3 mL single-patient-use Humalog KwikPen
- 3 mL single-patient-use Humalog Tempo Pen
- 3 mL single-patient-use Humalog Junior KwikPen
- 3 mL single-patient-use cartridges
Injection: 200 units per mL (U-200) clear and colorless solution available as:
- 3 mL single-patient-use Humalog KwikPen
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Never Share a HUMALOG Prefilled Pen, Cartridge, Reusable Pen Compatible with Lilly 3 mL Cartridges1, or Syringe Between Patients
HUMALOG prefilled pens, cartridges, and reusable pens compatible with Lilly 3 mL cartridges must never be shared between patients, even if the needle is changed. Patients using HUMALOG vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.3)] or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia [see Adverse Reactions (6)].
Make any changes to a patient's insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. For patients with type 2 diabetes, dosage adjustments of concomitant antidiabetic products may be needed.
5.3 Hypoglycemia
Hypoglycemia is the most common adverse reaction associated with insulins, including HUMALOG. Severe hypoglycemia can cause seizures, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery).
Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7)], or in patients who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia
The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulin preparations, the glucose lowering effect time course of HUMALOG may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature [see Clinical Pharmacology (12.2)]. Other factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to co-administered medication [see Drug Interactions (7)]. Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations (8.6, 8.7)].
Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
5.4 Hypoglycemia Due to Medication Errors
Accidental mix-ups between basal insulin products and other insulins, particularly rapid-acting insulins, have been reported. To avoid medication errors between HUMALOG and other insulins, instruct patients to always check the insulin label before each injection.
Do not transfer HUMALOG U-200 from the HUMALOG KwikPen to a syringe. The markings on the insulin syringe will not measure the dose correctly and can result in overdosage and severe hypoglycemia [see Dosage and Administration (2.1) and Warnings and Precautions (5.3)].
5.5 Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulin products, including HUMALOG. If hypersensitivity reactions occur, discontinue HUMALOG; treat per standard of care and monitor until symptoms and signs resolve [see Adverse Reactions (6.1)]. HUMALOG is contraindicated in patients who have had hypersensitivity reactions to HUMALOG or any of its excipients [see Contraindications (4)].
5.6 Hypokalemia
All insulin products, including HUMALOG, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations).
5.7 Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including HUMALOG, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
5.8 Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction
Malfunction of the insulin pump or insulin infusion set or insulin degradation can rapidly lead to hyperglycemia and ketoacidosis. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Interim subcutaneous injections with HUMALOG may be required. Patients using continuous subcutaneous insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure [see How Supplied/Storage and Handling (16.2) and Patient Counseling Information (17)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying designs, the adverse reaction rates reported in one clinical trial may not be easily compared with those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice.
The frequencies of Treatment-Emergent Adverse Events during HUMALOG clinical trials in patients with type 1 diabetes mellitus and type 2 diabetes mellitus are listed in the tables below.
Table 1: Treatment-Emergent Adverse Events in Patients with Type 1 Diabetes Mellitus (adverse events with frequency ≥5%) Events, n (%) Lispro
(n=81)Regular human insulin
(n=86)Flu syndrome 28 (34.6) 28 (32.6) Pharyngitis 27 (33.3) 29 (33.7) Rhinitis 20 (24.7) 25 (29.1) Headache 24 (29.6) 19 (22.1) Pain 16 (19.8) 14 (16.3) Cough increased 14 (17.3) 15 (17.4) Infection 11 (13.6) 18 (20.9) Nausea 5 (6.2) 13 (15.1) Accidental injury 7 (8.6) 10 (11.6) Surgical procedure 5 (6.2) 12 (14.0) Fever 5 (6.2) 10 (11.6) Abdominal pain 6 (7.4) 7 (8.1) Asthenia 6 (7.4) 7 (8.1) Bronchitis 6 (7.4) 6 (7.0) Diarrhea 7 (8.6) 5 (5.8) Dysmenorrhea 5 (6.2) 6 (7.0) Myalgia 6 (7.4) 5 (5.8) Urinary tract infection 5 (6.2) 4 (4.7) Table 2: Treatment-Emergent Adverse Events in Patients with Type 2 Diabetes Mellitus (adverse events with frequency ≥5%) Events, n (%) Lispro
(n=714)Regular human insulin
(n=709)Headache 83 (11.6) 66 (9.3) Pain 77 (10.8) 71 (10.0) Infection 72 (10.1) 54 (7.6) Pharyngitis 47 (6.6) 58 (8.2) Rhinitis 58 (8.1) 47 (6.6) Flu syndrome 44 (6.2) 58 (8.2) Surgical procedure 53 (7.4) 48 (6.8) Insulin initiation and intensification of glucose control
Intensification or rapid improvement in glucose control has been associated with a transitory, reversible ophthalmologic refraction disorder, worsening of diabetic retinopathy, and acute painful peripheral neuropathy. However, long-term glycemic control decreases the risk of diabetic retinopathy and neuropathy.
Lipodystrophy
Long-term use of insulin, including HUMALOG, can cause lipodystrophy at the site of repeated insulin injections or infusion. Lipodystrophy includes lipohypertrophy (thickening of adipose tissue) and lipoatrophy (thinning of adipose tissue), and may affect insulin absorption. Rotate insulin injection or infusion sites within the same region to reduce the risk of lipodystrophy [see Dosage and Administration (2.2)].
Weight gain
Weight gain can occur with insulin therapy, including HUMALOG, and has been attributed to the anabolic effects of insulin and the decrease in glucosuria.
Peripheral Edema
Insulin, including HUMALOG, may cause sodium retention and edema, particularly if previously poor metabolic control is improved by intensified insulin therapy.
Adverse Reactions with Continuous Subcutaneous Insulin Infusion (CSII) — HUMALOG U-100
In a 12-week, randomized, crossover study in adult patients with type 1 diabetes (n=39), the rates of catheter occlusions and infusion site reactions were similar for HUMALOG U-100 and regular human insulin treated patients (see Table 3).
Table 3: Catheter Occlusions and Infusion Site Reactions HUMALOG U-100
(n=38)Regular human insulin
(n=39)Catheter occlusions/month 0.09 0.10 Infusion site reactions 2.6% (1/38) 2.6% (1/39) In a randomized, 16-week, open-label, parallel design study of children and adolescents with type 1 diabetes, adverse event reports related to infusion-site reactions were similar for insulin lispro and insulin aspart (21% of 100 patients versus 17% of 198 patients, respectively). In both groups, the most frequently reported infusion site adverse events were infusion site erythema and infusion site reaction.
Allergic Reactions
Local Allergy — As with any insulin therapy, patients taking HUMALOG may experience redness, swelling, or itching at the site of the injection. These minor reactions usually resolve in a few days to a few weeks, but in some occasions, may require discontinuation of HUMALOG. In some instances, these reactions may be related to factors other than insulin, such as irritants in a skin cleansing agent or poor injection technique.
Systemic Allergy — Severe, life-threatening, generalized allergy, including anaphylaxis, may occur with any insulin, including HUMALOG. Generalized allergy to insulin may cause whole body rash (including pruritus), dyspnea, wheezing, hypotension, tachycardia, or diaphoresis.
In controlled clinical trials, pruritus (with or without rash) was seen in 17 patients receiving regular human insulin (n=2969) and 30 patients receiving HUMALOG (n=2944).
Localized reactions and generalized myalgias have been reported with injected metacresol, which is an excipient in HUMALOG [see Contraindications (4)].
Antibody Production
In large clinical trials with patients with type 1 (n=509) and type 2 (n=262) diabetes mellitus, anti-insulin antibody (insulin lispro-specific antibodies, insulin-specific antibodies, cross-reactive antibodies) formation was evaluated in patients receiving both regular human insulin and HUMALOG (including patients previously treated with human insulin and naive patients). As expected, the largest increase in the antibody levels occurred in patients new to insulin therapy. The antibody levels peaked by 12 months and declined over the remaining years of the study. These antibodies do not appear to cause deterioration in glycemic control or necessitate an increase in insulin dose. There was no statistically significant relationship between the change in the total daily insulin dose and the change in percent antibody binding for any of the antibody types.
6.2 Postmarketing Experience
HUMALOG U-100
The following additional adverse reactions have been identified during post-approval use of HUMALOG. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Medication errors in which other insulins have been accidentally substituted for HUMALOG have been identified during post-approval use [see Patient Counseling Information (17)].
Localized cutaneous amyloidosis at the injection site has occurred. Hyperglycemia has been reported with repeated insulin injections into areas of localized cutaneous amyloidosis; hypoglycemia has been reported with a sudden change to an unaffected injection site.
-
7 DRUG INTERACTIONS
7.1 Drugs That May Increase the Risk of Hypoglycemia
The risk of hypoglycemia associated with HUMALOG use may be increased when co-administered with antidiabetic agents, salicylates, sulfonamide antibiotics, monoamine oxidase inhibitors, fluoxetine, pramlintide, disopyramide, fibrates, pentoxifylline, ACE inhibitors, angiotensin II receptor blocking agents, and somatostatin analogs (e.g., octreotide). Dose adjustment and increased frequency of glucose monitoring may be required when HUMALOG is co-administered with these drugs.
7.2 Drugs That May Decrease the Blood Glucose Lowering Effect of HUMALOG
The glucose lowering effect of HUMALOG may be decreased when co-administered with corticosteroids, isoniazid, niacin, estrogens, oral contraceptives, phenothiazines, danazol, diuretics, sympathomimetic agents (e.g., epinephrine, albuterol, terbutaline), somatropin, atypical antipsychotics, glucagon, protease inhibitors, and thyroid hormones. Dose adjustment and increased frequency of glucose monitoring may be required when HUMALOG is co-administered with these drugs.
7.3 Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of HUMALOG
The glucose lowering effect of HUMALOG may be increased or decreased with co-administered with beta-blockers, clonidine, lithium salts, and alcohol. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. Dose adjustment and increased frequency of glucose monitoring may be required when HUMALOG is co-administered with these drugs.
7.4 Drugs That May Blunt Signs and Symptoms of Hypoglycemia
The signs and symptoms of hypoglycemia [see Warnings and Precautions (5.3)] may be blunted when beta-blockers, clonidine, guanethidine, and reserpine are co-administered with HUMALOG.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data with HUMALOG in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. Published studies with insulin lispro used during pregnancy have not reported an association between insulin lispro and the induction of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations).
Pregnant rats and rabbits were exposed to insulin lispro in animal reproduction studies during organogenesis. No adverse effects on embryo/fetal viability or morphology were observed in offspring of rats exposed to insulin lispro at a dose approximately 3 times the human subcutaneous dose of 1 unit insulin lispro/kg/day. No adverse effects on embryo/fetal development were observed in offspring of rabbits exposed to insulin lispro at doses up to approximately 0.2 times the human subcutaneous dose of 1 unit/kg/day (see Data).
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20-25% in women with a HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Human Data
Published data from retrospective studies and meta-analyses do not report an association with insulin lispro and major birth defects, miscarriage, or adverse maternal or fetal outcomes when insulin lispro is used during pregnancy. However, these studies cannot definitely establish or exclude the absence of any risk because of methodological limitations including small sample size, selection bias, confounding by unmeasured factors, and some lacking comparator groups.
Animal Data
In a combined fertility and embryo-fetal development study, female rats were given subcutaneous insulin lispro injections of 1, 5, and 20 units/kg/day (0.2, 0.8, and 3 times the human subcutaneous dose of 1 unit insulin lispro/kg/day, based on units/body surface area, respectively) from 2 weeks prior to cohabitation through Gestation Day 19. There were no adverse effects on female fertility, implantation, or fetal viability and morphology. However, fetal growth retardation was produced at the 20 units/kg/day-dose as indicated by decreased fetal weight and an increased incidence of fetal runts/litter.
In an embryo-fetal development study in pregnant rabbits, insulin lispro doses of 0.1, 0.25, and 0.75 unit/kg/day (0.03, 0.08, and 0.2 times the human subcutaneous dose of 1 unit insulin lispro/kg/day, based on units/body surface area, respectively) were injected subcutaneously on Gestation days 7 through 19. There were no adverse effects on fetal viability, weight, and morphology at any dose.
8.2 Lactation
Risk Summary
There are no data on the presence of HUMALOG in human milk, the effects on the breastfed infant, or the effect on milk production. One small published study reported that exogenous insulin was present in human milk. However, there is insufficient information to determine the effects of HUMALOG on the breastfed infant and no available information on the effects of HUMALOG on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for insulin, any potential adverse effects on the breastfed child from HUMALOG or from the underlying maternal condition.
8.4 Pediatric Use
HUMALOG is approved for use in children for subcutaneous daily injections [see Clinical Studies (14)]. Only the U-100 formulation of HUMALOG is approved for use in children by continuous subcutaneous infusion in insulin pumps. HUMALOG has not been studied in pediatric patients younger than 3 years of age. HUMALOG has not been studied in pediatric patients with type 2 diabetes.
As in adults, the dosage of HUMALOG must be individualized in pediatric patients based on metabolic needs and results of frequent monitoring of blood glucose.
8.5 Geriatric Use
Of the total number of subjects (n=2834) in eight clinical studies of HUMALOG, twelve percent (n=338) were 65 years of age or over. The majority of these had type 2 diabetes. HbA1c values and hypoglycemia rates did not differ by age. Pharmacokinetic/pharmacodynamic studies to assess the effect of age on the onset of HUMALOG action have not been performed.
8.6 Renal Impairment
Patients with renal impairment may be at increased risk of hypoglycemia and may require more frequent HUMALOG dose adjustment and more frequent blood glucose monitoring [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Patients with hepatic impairment may be at increased risk of hypoglycemia and may require more frequent HUMALOG dose adjustment and more frequent blood glucose monitoring [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Excess insulin administration may cause hypoglycemia and hypokalemia. Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise may be needed. More severe episodes with coma, seizure, or neurologic impairment may be treated with intramuscular/subcutaneous glucagon or concentrated intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after apparent clinical recovery. Hypokalemia must be corrected appropriately.
-
11 DESCRIPTION
Insulin lispro injection is a rapid-acting human insulin analog. Insulin lispro is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli. Insulin lispro differs from human insulin in that the amino acid proline at position B28 is replaced by lysine and the lysine in position B29 is replaced by proline. Chemically, it is Lys(B28), Pro(B29) human insulin analog and has the empirical formula C257H383N65O77S6 and a molecular weight of 5808, both identical to that of human insulin.
HUMALOG has the following primary structure:
HUMALOG (insulin lispro injection) is a sterile, aqueous, clear, and colorless solution for subcutaneous or intravenous use. Each milliliter of HUMALOG U-100 contains 100 units of insulin lispro, dibasic sodium phosphate (1.88 mg), glycerin (16 mg), Metacresol (3.15 mg), zinc oxide (content adjusted to provide 0.0197 mg zinc ion), trace amounts of phenol, and Water for Injection, USP. Each milliliter of HUMALOG U-200 contains 200 units of insulin lispro, glycerin (16 mg), Metacresol (3.15 mg), tromethamine (5 mg), zinc oxide (content adjusted to provide 0.046 mg zinc ion), trace amounts of phenol, and Water for Injection, USP. Insulin lispro has a pH of 7.0 to 7.8.
The pH is adjusted by addition of aqueous solutions of hydrochloric acid 10% and/or sodium hydroxide 10%.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Regulation of glucose metabolism is the primary activity of insulins and insulin analogs, including insulin lispro. Insulins lower blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulins inhibit lipolysis and proteolysis, and enhance protein synthesis.
12.2 Pharmacodynamics
HUMALOG has been shown to be equipotent to human insulin on a molar basis. One unit of HUMALOG has the same glucose-lowering effect as one unit of regular human insulin. Studies in normal volunteers and patients with diabetes demonstrated that HUMALOG has a more rapid onset of action and a shorter duration of activity than regular human insulin when given subcutaneously.
The time course of action of insulin and insulin analogs, such as HUMALOG, may vary considerably in different individuals or within the same individual. The parameters of HUMALOG activity (time of onset, peak time, and duration) as designated in Figure 1 should be considered only as general guidelines. The rate of insulin absorption, and consequently the onset of activity are known to be affected by the site of injection, exercise, and other variables [see Warnings and Precautions (5.2)].
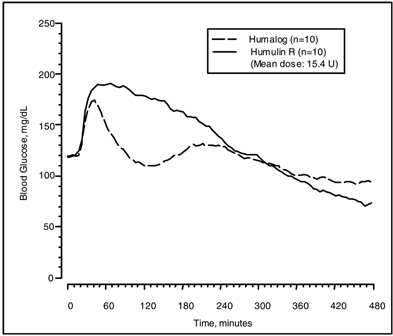
Figure 1: Blood Glucose Levels After Subcutaneous Injection of Regular Human Insulin or HUMALOG (0.2 unit/kg) Immediately Before a High Carbohydrate Meal in 10 Patients with Type 1 Diabetesa.
a Baseline insulin concentration was maintained by infusion of 0.2 mU/min/kg human insulin.
Intravenous Administration of HUMALOG U-100 — The glucose lowering effect of intravenously administered HUMALOG was tested in 21 patients with type 1 diabetes. For the study, the patients' usual doses of insulin were held and blood glucose concentrations were allowed to reach a stable range of 200 to 260 mg/dL during a one to three hours run-in phase. The run-in phase was followed by a 6-hour assessment phase. During the assessment phase, patients received intravenous HUMALOG at an initial infusion rate of 0.5 units/hour. The infusion rate of HUMALOG could be adjusted at regular timed intervals to achieve and maintain blood glucose concentrations between 100 to 160 mg/dL.
The mean blood glucose levels during the assessment phase for patients on HUMALOG therapy are summarized below in Table 4. All patients achieved the targeted glucose range at some point during the 6-hour assessment phase. At the endpoint, blood glucose was within the target range (100 to 160 mg/dL) for 17 of 20 patients treated with HUMALOG. The average time (±SE) required to attain near normoglycemia was 129 ± 14 minutes for HUMALOG.
Table 4: Mean Blood Glucose Concentrations (mg/dL) During Intravenous Infusions of HUMALOG U-100 a Results shown as mean ± SD
Time from Start of Infusion (minutes) Mean Blood Glucose (mg/dL) Intravenousa 0 224 ± 16 30 205 ± 21 60 195 ± 20 120 165 ± 26 180 140 ± 26 240 123 ± 20 300 120 ± 27 360 122 ± 25 The pharmacodynamics of a single 20 unit dose of HUMALOG U-200 administered subcutaneously were compared to the pharmacodynamics of a single 20 unit dose of HUMALOG U-100 administered subcutaneously in a euglycemic clamp study enrolling healthy subjects. In this study, the overall, maximum, and time to maximum glucose lowering effect were similar between HUMALOG U-200 and HUMALOG U-100. The mean area under the glucose infusion rate curves (measure of overall pharmacodynamic effect) were 125 g and 126 g for HUMALOG U-200 and HUMALOG U-100, respectively. The maximum glucose infusion rate was 534 mg/min and 559 mg/min and the corresponding median time (min, max) to maximum effect were 2.8 h (0.5 h – 6.3 h) and 2.4 h (0.5 h – 4.7 h) for HUMALOG U-200 and HUMALOG U-100, respectively.
12.3 Pharmacokinetics
Absorption and Bioavailability — Studies in healthy volunteers and patients with diabetes demonstrated that HUMALOG is absorbed more quickly than regular human insulin. In healthy volunteers given subcutaneous doses of HUMALOG ranging from 0.1 to 0.4 unit/kg, peak serum levels were seen 30 to 90 minutes after dosing. When healthy volunteers received equivalent doses of regular human insulin, peak insulin levels occurred between 50 to 120 minutes after dosing. Similar results were seen in patients with type 1 diabetes (see Figure 2).
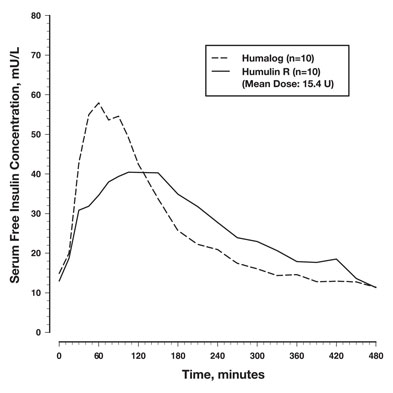
Figure 2: Serum HUMALOG and Insulin Levels After Subcutaneous Injection of Regular Human Insulin or HUMALOG (0.2 unit/kg) Immediately Before a High Carbohydrate Meal in 10 Patients with Type 1 Diabetesa.
a Baseline insulin concentration was maintained by infusion of 0.2 mU/min/kg human insulin.
HUMALOG U-100 was absorbed at a consistently faster rate than regular human insulin in healthy male volunteers given 0.2 unit/kg at abdominal, deltoid, or femoral subcutaneous sites. After HUMALOG was administered in the abdomen, serum drug levels were higher and the duration of action was slightly shorter than after deltoid or thigh administration. Bioavailability of HUMALOG is similar to that of regular human insulin. The absolute bioavailability after subcutaneous injection ranges from 55% to 77% with doses between 0.1 to 0.2 unit/kg, inclusive.
The results of a study in healthy subjects demonstrated that HUMALOG U-200 is bioequivalent to HUMALOG U-100 following administration of a single 20 unit dose.
The mean observed area under the serum insulin concentration-time curve from time zero to infinity was 2360 pmol hr/L and 2390 pmol hr/L for HUMALOG U-200 and HUMALOG U-100, respectively. The corresponding mean peak serum insulin concentration was 795 pmol/L and 909 pmol/L for HUMALOG U-200 and HUMALOG U-100, respectively. The median time to maximum concentration was 1.0 hour for both formulations.
Distribution — When administered intravenously as bolus injections of 0.1 and 0.2 U/kg dose in two separate groups of healthy subjects, the mean volume of distribution of HUMALOG appeared to decrease with increase in dose (1.55 and 0.72 L/kg, respectively) in contrast to that of regular human insulin for which, the volume of distribution was comparable across the two dose groups (1.37 and 1.12 L/kg for 0.1 and 0.2 U/kg dose, respectively).
Metabolism — Human metabolism studies have not been conducted. However, animal studies indicate that the metabolism of HUMALOG is identical to that of regular human insulin.
Elimination — After subcutaneous administration of HUMALOG, the t1/2 is shorter than that of regular human insulin (1 versus 1.5 hours, respectively). When administered intravenously, HUMALOG and regular human insulin demonstrated similar dose-dependent clearance, with a mean clearance of 21.0 mL/min/kg and 21.4 mL/min/kg, respectively (0.1 unit/kg dose), and 9.6 mL/min/kg and 9.4 mL/min/kg, respectively (0.2 unit/kg dose). Accordingly, HUMALOG demonstrated a mean t1/2 of 0.85 hours (51 minutes) and 0.92 hours (55 minutes), respectively for 0.1 unit/kg and 0.2 unit/kg doses, and regular human insulin mean t1/2 was 0.79 hours (47 minutes) and 1.28 hours (77 minutes), respectively for 0.1 unit/kg and 0.2 unit/kg doses.
Specific Populations
The effects of age, gender, race, obesity, pregnancy, or smoking on the pharmacokinetics of HUMALOG have not been studied.
Renal Impairment — Type 2 diabetic patients with varying degree of renal impairment showed no difference in pharmacokinetics of regular insulin and HUMALOG. However, the sensitivity of the patients to insulin did change, with an increased response to insulin as the renal function declined. Some studies with human insulin have shown increased circulating levels of insulin in patients with renal impairment. Careful glucose monitoring and dose adjustments of insulin, including HUMALOG, may be necessary in patients with renal dysfunction.
Hepatic Impairment — Type 2 diabetic patients with impaired hepatic function showed no effect on the pharmacokinetics of HUMALOG as compared to patients with no hepatic dysfunction. However, some studies with human insulin have shown increased circulating levels of insulin in patients with liver failure. Careful glucose monitoring and dose adjustments of insulin, including HUMALOG, may be necessary in patients with hepatic dysfunction.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard 2-year carcinogenicity studies in animals have not been performed. In Fischer 344 rats, a 12-month repeat-dose toxicity study was conducted with insulin lispro at subcutaneous doses of 20 and 200 units/kg/day (approximately 3 and 32 times the human subcutaneous dose of 1 unit/kg/day, based on units/body surface area). Insulin lispro did not produce important target organ toxicity including mammary tumors at any dose.
Insulin lispro was not mutagenic in the following genetic toxicity assays: bacterial mutation, unscheduled DNA synthesis, mouse lymphoma, chromosomal aberration and micronucleus assays.
Male fertility was not compromised when male rats given subcutaneous insulin lispro injections of 5 and 20 units/kg/day (0.8 and 3 times the human subcutaneous dose of 1 unit/kg/day, based on units/body surface area) for 6 months were mated with untreated female rats. In a combined fertility, perinatal, and postnatal study in male and female rats given 1, 5, and 20 units/kg/day subcutaneously (0.2, 0.8, and 3 times the human subcutaneous dose of 1 unit/kg/day, based on units/body surface area), mating and fertility were not adversely affected in either gender at any dose.
-
14 CLINICAL STUDIES
The safety and efficacy of HUMALOG U-100 were studied in children, adolescent, and adult patients with type 1 diabetes (n=789) and adult patients with type 2 diabetes (n=722).
14.1 Type 1 Diabetes – Adults and Adolescents
A 12-month, randomized, parallel, open-label, active-controlled study was conducted in patients with type 1 diabetes to assess the safety and efficacy of HUMALOG (n=81) compared with Humulin® R [insulin human injection (100 units per mL)] (n=86). HUMALOG was administered by subcutaneous injection immediately prior to meals and Humulin R was administered 30 to 45 minutes before meals. Humulin® U [ULTRALENTE® human insulin (rDNA origin) extended zinc suspension] was administered once or twice daily as the basal insulin. There was a 2- to 4-week run-in period with Humulin R and Humulin U before randomization. Most patients were Caucasian (97%). Forty-seven percent of the patients were male. The mean age was 31 years (range 12 to 70 years). Glycemic control, the total daily doses of HUMALOG and Humulin R, and the incidence of severe hypoglycemia (as determined by the number of events that were not self-treated) were similar in the two treatment groups. There were no episodes of diabetic ketoacidosis in either treatment group.
Table 5: Type 1 Diabetes Mellitus – Adults and Adolescents a Values are Mean ± SD
b Severe hypoglycemia refers to hypoglycemia for which patients were not able to self-treat.
Treatment Duration
Treatment in Combination with:12 months
Humulin UHUMALOG Humulin R N 81 86 Baseline HbA1c (%)a 8.2 ± 1.4 8.3 ± 1.7 Change from baseline HbA1c (%)a -0.1 ± 0.9 0.1 ± 1.1 Treatment Difference in HbA1c Mean (95% confidence interval) 0.4 (0.0, 0.8) Baseline short-acting insulin dose (units/kg/day) 0.3 ± 0.1 0.3 ± 0.1 End-of-Study short-acting insulin dose (units/kg/day) 0.3 ± 0.1 0.3 ± 0.1 Change from baseline short-acting insulin dose (units/kg/day) 0.0 ± 0.1 0.0 ± 0.1 Baseline Body weight (kg) 72 ± 12.7 71 ± 11.3 Weight change from baseline (kg) 1.4 ± 3.6 1.0 ± 2.6 Patients with severe hypoglycemia (n, %)b 14 (17%) 18 (21%) 14.2 Type 2 Diabetes – Adults
A 6-month randomized, crossover, open-label, active-controlled study was conducted in insulin-treated patients with type 2 diabetes (n=722) to assess the safety and efficacy of HUMALOG for 3 months followed by Humulin R for 3 months or the reverse sequence. HUMALOG was administered by subcutaneous injection immediately before meals and Humulin R was administered 30 to 45 minutes before meals. Humulin® N [NPH human insulin (rDNA origin) isophane suspension] or Humulin U was administered once or twice daily as the basal insulin. All patients participated in a 2- to 4-week run-in period with Humulin R and Humulin N or Humulin U. Most of the patients were Caucasian (88%), and the numbers of men and women in each group were approximately equal. The mean age was 58.6 years (range 23.8 to 85 years). The average body mass index (BMI) was 28.2 kg/m2. During the study, the majority of patients used Humulin N (84%) compared with Humulin U (16%) as their basal insulin. The reductions from baseline in HbA1c and the incidence of severe hypoglycemia (as determined by the number of events that were not self-treated) were similar between the two treatments from the combined groups (see Table 6).
Table 6: Type 2 Diabetes Mellitus — Adults a Values are Mean ± SD
b Severe hypoglycemia refers to hypoglycemia for which patients were not able to self-treat.
End point Baseline HUMALOG
+
BasalHumulin R
+
BasalHbA1c (%)a 8.9 ± 1.7 8.2 ± 1.3 8.2 ± 1.4 Change from baseline HbA1c (%)a — -0.7 ± 1.4 -0.7 ± 1.3 Short-acting insulin dose (units/kg/day)a 0.3 ± 0.2 0.3 ± 0.2 0.3 ± 0.2 Change from baseline short-acting insulin dose (units/kg/day)a — 0.0 ± 0.1 0.0 ± 0.1 Body weight (kg)a 80 ± 15 81 ± 15 81 ± 15 Weight change from baseline — 0.8 ± 2.7 0.9 ± 2.6 Patients with severe hypoglycemia (n, %)b — 15 (2%) 16 (2%) 14.3 Type 1 Diabetes – Pediatric and Adolescents
An 8-month, crossover study of adolescents with type 1 diabetes (n=463), aged 9 to 19 years, compared two subcutaneous multiple-dose treatment regimens: HUMALOG or Humulin R, both administered with Humulin N (NPH human insulin) as the basal insulin. HUMALOG achieved glycemic control comparable to Humulin R, as measured by HbA1c (see Table 7), and both treatment groups had a comparable incidence of hypoglycemia. In a 9-month, crossover study of prepubescent children (n=60) with type 1 diabetes, aged 3 to 11 years, HUMALOG administered immediately before meals, HUMALOG administered immediately after meals and Humulin R administered 30 minutes before meals resulted in similar glycemic control, as measured by HbA1c, and incidence of hypoglycemia, regardless of treatment group.
Table 7: Pediatric Subcutaneous Administration of HUMALOG in Type 1 Diabetes a Values are Mean ± SD
b Severe hypoglycemia refers to hypoglycemia that required glucagon or glucose injection or resulted in coma.
End point Baseline HUMALOG
+
NPHHumulin R
+
NPHHbA1c (%)a 8.6 ± 1.5 8.7 ± 1.5 8.7 ± 1.6 Change from baseline HbA1c (%)a — 0.1 ± 1.1 0.1 ± 1.3 Short-acting insulin dose (units/kg/day)a 0.5 ± 0.2 0.5 ± 0.2 0.5 ± 0.2 Change from baseline short-acting insulin dose (units/kg/day)a — 0.01 ± 0.1 -0.01 ± 0.1 Body weight (kg)a 59.1 ± 13.1 61.1 ± 12.7 61.4 ± 12.9 Weight change from baseline (kg)a — 2.0 ± 3.1 2.3 ± 3.0 Patients with severe hypoglycemia (n, %)b — 5 (1.1%) 5 (1.1%) Diabetic ketoacidosis (n, %) — 11 (2.4%) 9 (1.9%) 14.4 Type 1 Diabetes – Adults Continuous Subcutaneous Insulin Infusion
To evaluate the administration of HUMALOG U-100 via external insulin pumps, two open-label, crossover design studies were performed in patients with type 1 diabetes. One study involved 39 patients, ages 19 to 58 years, treated for 24 weeks with HUMALOG or regular human insulin. After 12 weeks of treatment, the mean HbA1c values decreased from 7.8% to 7.2% in the HUMALOG-treated patients and from 7.8% to 7.5% in the regular human insulin-treated patients. Another study involved 60 patients (mean age 39, range 15 to 58 years) treated for 24 weeks with either HUMALOG or buffered regular human insulin. After 12 weeks of treatment, the mean HbA1c values decreased from 7.7% to 7.4% in the HUMALOG-treated patients and remained unchanged from 7.7% in the buffered regular human insulin-treated patients. Rates of hypoglycemia were comparable between treatment groups in both studies.
14.5 Type 1 Diabetes – Pediatric Continuous Subcutaneous Insulin Infusion
A randomized, 16-week, open-label, parallel design, study of children and adolescents with type 1 diabetes (n=298) aged 4 to 18 years compared two subcutaneous infusion regimens administered via an external insulin pump: insulin aspart (n=198) or HUMALOG U-100 (n=100). These two treatments resulted in comparable changes from baseline in HbA1c and comparable rates of hypoglycemia after 16 weeks of treatment (see Table 8). Infusion site reactions were similar between groups.
Table 8: Pediatric Insulin Pump Study in Type 1 Diabetes (16 weeks; n=298) a Values are Mean ± SD
b Severe hypoglycemia refers to hypoglycemia associated with central nervous system symptoms and requiring the intervention of another person or hospitalization.
HUMALOG Aspart N 100 198 Baseline HbA1c (%)a 8.2 ± 0.8 8.0 ± 0.9 Change from Baseline HbA1c (%) -0.1 ± 0.7 -0.1 ± 0.8 Treatment Difference in HbA1c, Mean (95% confidence interval) 0.1 (-0.3, 0.1) Baseline insulin dose (units/kg/24 hours)a 0.9 ± 0.3 0.9 ± 0.3 End-of-Study insulin dose (units/kg/24 hours)a 0.9 ± 0.2 0.9 ± 0.2 Patients with severe hypoglycemia (n, %)b 8 (8%) 19 (10%) Diabetic ketoacidosis (n, %) 0 (0) 1 (0.5%) Baseline body weight (kg)a 55.5 ± 19.0 54.1 ± 19.7 Weight Change from baseline (kg)a 1.6 ± 2.1 1.8 ± 2.1 -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
HUMALOG (insulin lispro injection) is a clear and colorless solution available as:
a Tempo Pen contains a component that allows for data connectivity when used with a compatible transmitter.
HUMALOG Total Volume Concentration Total Units Available in Presentation NDC Number Package Size U-100 multiple-dose vial 10 mL 100 units/mL 1000 units 0002-7510-01 1 vial U-100 multiple-dose vial 3 mL 100 units/mL 300 units 0002-7510-17 1 vial U-100 single-patient-use cartridge1 3 mL 100 units/mL 300 units 0002-7516-59 5 cartridges U-100 single-patient-use KwikPen 3 mL 100 units/mL 300 units 0002-8799-59 5 pens U-100 single-patient-use Tempo Pena 3 mL 100 units/mL 300 units 0002-8213-05 5 pens U-100 single-patient-use Junior KwikPen 3 mL 100 units/mL 300 units 0002-7714-59 5 pens U-200 single-patient-use KwikPen 3 mL 200 units/mL 600 units 0002-7712-27 2 pens The U-100 KwikPen, U-100 Tempo Pen, and U-200 KwikPen dial in 1 unit increments. The U-100 Junior KwikPen dials in 0.5 unit increments.
Each prefilled pen, cartridge, and reusable pen compatible with Lilly 3 mL cartridges is for single-patient-use only. HUMALOG prefilled pens, cartridges, and reusable pens compatible with Lilly 3 mL cartridges must never be shared between patients, even if the needle is changed. Patients using HUMALOG vials must never share needles or syringes with another person.
16.2 Storage and Handling
Dispense in the original sealed carton with the enclosed Instructions for Use.
Do not use after the expiration date.
Unopened HUMALOG should be stored in a refrigerator (36° to 46°F [2° to 8°C]), but not in the freezer. Do not use HUMALOG if it has been frozen. In-use HUMALOG vials, cartridges, and HUMALOG prefilled pens should be stored at room temperature, below 86°F (30°C) and must be used within 28 days or be discarded, even if they still contain HUMALOG. Protect from direct heat and light. See table below:
Not In-Use (Unopened) Room Temperature (Below 86°F [30°C]) Not In-Use (Unopened) Refrigerated In-Use (Opened) Room Temperature, (Below 86°F [30°C]) HUMALOG U-100 10 mL multiple-dose vial 28 days Until expiration date 28 days, refrigerated/room temperature. 3 mL multiple-dose vial 28 days Until expiration date 28 days, refrigerated/room temperature. 3 mL single-patient-use cartridge 28 days Until expiration date 28 days, Do not refrigerate. 3 mL single-patient-use Humalog KwikPen 28 days Until expiration date 28 days, Do not refrigerate. 3 mL single-patient-use Humalog Tempo Pen 28 days Until expiration date 28 days, Do not refrigerate. 3 mL single-patient-use Humalog Junior KwikPen 28 days Until expiration date 28 days, Do not refrigerate. HUMALOG U-200 3 mL single-patient use Humalog KwikPen 28 days Until expiration date 28 days, Do not refrigerate. Use in an External Insulin Pump — Change the HUMALOG U-100 in the reservoir at least every 7 days, change the infusion sets and the infusion set insertion site at least every 3 days or after exposure to temperatures that exceed 98.6°F (37°C). A HUMALOG 3 mL cartridge used in the D-Tron pumps should be discarded after 7 days, even if it still contains HUMALOG. However, as with other external insulin pumps, the infusion set should be replaced and a new infusion set insertion site should be selected at least every 3 days.
16.3 Preparation and Handling
Diluted HUMALOG U-100 for Subcutaneous Injection — HUMALOG may be diluted with Sterile Diluent for HUMALOG for subcutaneous injection. Diluting one part HUMALOG to nine parts diluent will yield a concentration one-tenth that of HUMALOG (equivalent to U-10). Diluting one part HUMALOG to one part diluent will yield a concentration one-half that of HUMALOG (equivalent to U-50).
16.4 Admixture for Intravenous Administration
Infusion bags prepared with HUMALOG U-100 are stable when stored in a refrigerator (2° to 8°C [36° to 46°F]) for 48 hours and then may be used at room temperature for up to an additional 48 hours [see Dosage and Administration (2.2)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Never Share a HUMALOG Prefilled Pen, Cartridge, Reusable Pen Compatible with Lilly 3 mL Cartridges, or Syringe Between Patients
Advise patients that they must never share a HUMALOG prefilled pen, cartridge, or reusable pen compatible with Lilly 3 mL cartridges with another person, even if the needle is changed. Advise patients using HUMALOG vials not to share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens [see Warnings and Precautions (5.1)].
Hyperglycemia or Hypoglycemia
Instruct patients on self-management procedures including glucose monitoring, proper injection technique, and management of hypoglycemia and hyperglycemia, especially at initiation of HUMALOG therapy. Instruct patients on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, and skipped meals. Instruct patients on the management of hypoglycemia.
Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia. Advise patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia to use caution when driving or operating machinery [see Warnings and Precautions (5.3)].
Advise patients that changes in insulin regimen can predispose to hyperglycemia or hypoglycemia and that changes in insulin regimen should be made under close medical supervision [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions have occurred with HUMALOG. Inform patients on the symptoms of hypersensitivity reactions [see Warnings and Precautions (5.5)].
Medication Errors
Instruct patients to always check the insulin label before each injection to avoid mix-ups between insulin products.
Inform patients that HUMALOG U-200 contains 2 times as much insulin in 1 mL as HUMALOG U-100.
Inform patients that the HUMALOG U-200 KwikPen dose window shows the number of units of HUMALOG U-200 to be injected and that no dose conversion is required.
Instruct patients to NOT transfer HUMALOG U-200 from the HUMALOG KwikPen to a syringe. The markings on the syringe will not measure the dose correctly and this can result in overdosage and severe hypoglycemia.
Administration Instruction for HUMALOG U-200
Instruct patients to NOT mix HUMALOG U-200 with any other insulin.
Instructions For Patients Using Continuous Subcutaneous Insulin Pumps
Patients using external pump infusion therapy should be trained appropriately.
The following insulin pumps have been tested in HUMALOG clinical trials conducted by Eli Lilly and Company.
- Disetronic® H-Tron® plus V100, D-Tron® and D-Tronplus® with Disetronic Rapid infusion sets2
- MiniMed® Models 506, 507 and 508 and Polyfin® infusion sets3
HUMALOG is recommended for use in pump systems suitable for insulin infusion such as MiniMed, Disetronic, and other equivalent pumps. Before using HUMALOG in a pump system, read the pump label to make sure the pump is indicated for continuous delivery of fast-acting insulin. HUMALOG is recommended for use in any reservoir and infusion sets that are compatible with insulin and the specific pump. Please see recommended reservoir and infusion sets in the pump manual. Do not use HUMALOG U-200 in an external insulin pump.
To avoid insulin degradation, infusion set occlusion, and loss of the preservative (metacresol), insulin in the reservoir should be replaced at least every 7 days; infusion sets and infusion set insertion sites should be changed at least every 3 days.
Insulin exposed to temperatures higher than 98.6°F (37°C) should be discarded. The temperature of the insulin may exceed ambient temperature when the pump housing, cover, tubing or sport case is exposed to sunlight or radiant heat. Infusion sites that are erythematous, pruritic, or thickened should be reported to the healthcare professional, and a new site selected because continued infusion may increase the skin reaction or alter the absorption of HUMALOG.
Pump or infusion set malfunctions or insulin degradation can lead to rapid hyperglycemia and ketosis. This is especially pertinent for rapid acting insulin analogs that are more rapidly absorbed through skin and have a shorter duration of action. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Problems include pump malfunction, infusion set occlusion, leakage, disconnection or kinking, and degraded insulin. Less commonly, hypoglycemia from pump malfunction may occur. If these problems cannot be promptly corrected, patients should resume therapy with subcutaneous insulin injection and contact their healthcare professionals [see Dosage and Administration (2.2) and How Supplied/Storage and Handling (16.2)].
____________
1 3 mL cartridge is for use in Eli Lilly and Company's HumaPen® Luxura® HD insulin delivery device, and Disetronic
D-TRON® and D-TRON® Plus pumps.Humalog®, Humalog KwikPen®, Humalog Tempo Pen™ ,Humalog® Junior KwikPen®, HumaPen®, HumaPen® Luxura® and HumaPen® Luxura® HD are trademarks of Eli Lilly and Company.
2 Disetronic®, H-Tron®, D-Tron®, and D-Tronplus® are registered trademarks of Roche Diagnostics GmbH.
3 MiniMed® and Polyfin® are registered trademarks of MiniMed, Inc.
Other product and company names may be the trademarks of their respective owners.
Literature revised November 2019
Marketed by: Lilly USA, LLC, Indianapolis, IN 46285, USA
www.humalog.com
Copyright © 1996, 2019, Eli Lilly and Company. All rights reserved.
LOG-0009-USPI-20191115
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: November 2019
Patient Information
HUMALOG® (HU-ma-log)
insulin lispro injection
U-100 (100 units per mL)
for subcutaneous or intravenous useDo not share your Humalog prefilled pens, cartridges, reusable pen compatible with Lilly 3 mL cartridges, needles, or syringes with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them. What is HUMALOG?
- HUMALOG is a man-made fast-acting insulin used to control high blood sugar in adults and children with diabetes mellitus.
- It is not known if HUMALOG is safe and effective in children younger than 3 years of age or when used to treat children with type 2 diabetes mellitus.
Who should not take HUMALOG?
Do not take HUMALOG if you:
- are having an episode of low blood sugar (hypoglycemia).
- have an allergy to HUMALOG or any of the ingredients in HUMALOG. See the end of this Patient Information leaflet for a complete list of ingredients in HUMALOG.
What should I tell my healthcare provider before taking HUMALOG?
Before taking HUMALOG, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney or liver problems.
- take any other medicines, especially ones called TZDs (thiazolidinediones).
- have heart failure or other heart problems. If you have heart failure, it may get worse while you take TZDs with HUMALOG.
- are pregnant or plan to become pregnant. Talk with your healthcare provider about the best way to control your blood sugar if you plan to become pregnant or while you are pregnant.
- are breastfeeding or plan to breastfeed. Talk with your healthcare provider about the best way to feed your baby while taking HUMALOG.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Before you start taking HUMALOG, talk to your healthcare provider about low blood sugar and how to manage it.How should I take HUMALOG?
- Read the Instructions for Use that comes with your HUMALOG.
- Take HUMALOG exactly as your healthcare provider tells you to. Your healthcare provider should tell you how much HUMALOG to take and when to take it.
- HUMALOG starts acting fast. Inject HUMALOG within 15 minutes before or right after you eat a meal.
- Know the type, strength and amount of insulin you take. Do not change the type or amount of insulin you take unless your healthcare provider tells you to. The amount of insulin and the best time for you to take your insulin may need to change if you take different types of insulin.
- Check your insulin label each time you give your injection to make sure you are taking the correct insulin.
- Inject HUMALOG under the skin (subcutaneously) of your stomach area, buttocks, upper legs or upper arms, or by continuous infusion under the skin (subcutaneously) through an insulin pump into an area of your body recommended in the instructions that come with your insulin pump.
-
Change (rotate) your injection sites within the area you choose with each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not use the exact same spot for each injection.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Always use a new needle for each injection to help prevent infections and blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection or get a serious infection from them.
-
Check your blood sugar levels. Ask your healthcare provider what your blood sugars should be and when you should check your blood sugar levels.
Keep HUMALOG and all medicines out of the reach of children. Your dose of HUMALOG may need to change because of a:
- change in physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, or because of other medicines you take.
What should I avoid while taking HUMALOG?
While taking HUMALOG do not:
- drive or operate heavy machinery, until you know how HUMALOG affects you.
- drink alcohol or take prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of HUMALOG?
HUMALOG may cause serious side effects that can lead to death, including:
-
low blood sugar (hypoglycemia). Signs and symptoms of low blood sugar may include:
- dizziness or light-headedness
- sweating
- confusion
- headache
- blurred vision
- slurred speech
- shakiness
- fast heartbeat
- anxiety, irritability or mood changes
- hunger
Your healthcare provider may prescribe a glucagon emergency kit so that someone else can give you glucagon if your blood sugar becomes too low (severe hypoglycemia) and you are unable to take sugar by mouth.
-
serious allergic reactions (whole body allergic reaction). Get medical help right away, if you have any of these signs or symptoms of a severe allergic reaction:
- a rash over your whole body
- trouble breathing
- a fast heartbeat
- sweating
- feel faint
- low potassium in your blood (hypokalemia).
- heart failure. Taking certain diabetes pills called thiazolidinediones or “TZDs” with HUMALOG may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with HUMALOG. Your healthcare provider should monitor you closely while you are taking TZDs with HUMALOG. Tell your healthcare provider if you have any new or worse symptoms of heart failure including:
- shortness of breath
- swelling of your ankles or feet
- sudden weight gain
Treatment with TZDs and HUMALOG may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
Get emergency medical help if you have:- trouble breathing
- shortness of breath
- fast heartbeat
- swelling of your face, tongue, or throat
- sweating
- extreme drowsiness
- dizziness
- confusion
The most common side effects of HUMALOG include: - low blood sugar (hypoglycemia)
- reactions at your injection site
- skin thickening or pits at the injection site (lipodystrophy)
- weight gain
- swelling in your hands or feet
- itching
- rash
These are not all the possible side effects of HUMALOG. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. General information about the safe and effective use of HUMALOG.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not take HUMALOG for a condition for which it was not prescribed. Do not give HUMALOG to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about HUMALOG. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about HUMALOG that is written for health professionals.What are the ingredients in HUMALOG?
Active ingredient: insulin lispro
Inactive ingredients: dibasic sodium phosphate, glycerin, metacresol, zinc oxide (zinc ion), trace amounts of phenol and Water for Injection, USP.
Humalog® is a registered trademark of Eli Lilly and Company.
Marketed by: Lilly USA, LLC, Indianapolis, IN 46285, USA
Copyright © 1996, 2019, Eli Lilly and Company. All rights reserved.
For more information, go to www.humalog.com or call 1-800-545-5979.LOG-0006-PPI-20191115
-
INSTRUCTIONS FOR USE
Instructions for Use
HUMALOG® (HU-ma-log)
(insulin lispro injection)Read this Instructions for Use before you start taking HUMALOG and each time you get a new vial. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your needles or syringes with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
Supplies needed to give your injection
- a multiple-dose HUMALOG vial
- a U-100 insulin syringe and needle
- 2 alcohol swabs
- gauze
- 1 sharps container for throwing away used needles and syringes. See “Disposing of used needles and syringes” at the end of these instructions.
Preparing your HUMALOG dose
- Wash your hands with soap and water.
- Check the HUMALOG label to make sure you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- HUMALOG should look clear and colorless. Do not use HUMALOG if it is thick, cloudy, or colored, or if you see lumps or particles in it.
- Do not use HUMALOG past the expiration date printed on the label or 28 days after you first use it.
- Always use a new syringe and needle for each injection to prevent infections and blocked needles. Do not reuse or share your syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
If you use HUMALOG with NPH insulin:
- NPH insulin is the only type of insulin that can be mixed with HUMALOG. Do not mix HUMALOG with any other type of insulin.
- HUMALOG should be drawn up into the syringe first, before you draw up your NPH insulin. Talk to your healthcare provider if you are not sure about the right way to mix HUMALOG and NPH insulin.
- Give your injection right away.
Giving your HUMALOG Injection with a syringe
- Inject your insulin exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you should pinch the skin before injecting.
- HUMALOG starts acting fast, so give your injection within 15 minutes before or right after you eat a meal.
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
Giving your HUMALOG using an insulin pump
- HUMALOG should be given into an area of your body recommended in the instructions that come with your insulin pump.
- Change (rotate) your insertion site every 3 days.
- Change (rotate) your insertion sites within the area you choose for each insertion to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the insertion sites. Do not insert into the exact same spot for each insertion. Do not insert where the skin has pits, is thickened, or has lumps. Do not insert where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Change the insulin in the reservoir at least every 7 days, even if you have not used all of the insulin.
- Do not dilute or mix HUMALOG with any other type of insulin in your insulin pump.
- See your insulin pump manual for instructions or talk to your healthcare provider.
Disposing of used needles and syringes
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- - made of a heavy-duty plastic,
- - can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- - upright and stable during use,
- - leak-resistant, and
- - properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at:
http://www.fda.gov/safesharpsdisposal.- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store HUMALOG?
All unopened vials:
- Store all unopened vials in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze. Do not use if HUMALOG has been frozen.
- Keep away from heat and out of direct light.
- Unopened vials can be used until the expiration date on the carton and label, if they have been stored in the refrigerator.
- Unopened vials should be thrown away after 28 days, if they are stored at room temperature.
After vials have been opened:
- Store opened vials in the refrigerator or at room temperature below 86°F (30°C) for up to 28 days.
- Keep vials away from heat and out of direct light.
- Throw away all opened vials after 28 days of use, even if there is insulin left in the vial.
Keep HUMALOG vials, syringes, needles and all medicines out of the reach of children.
If you have any questions or problems with your HUMALOG, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) or call your healthcare provider for help. For more information on HUMALOG and insulin, go to www.humalog.com.
Scan this code to launch the humalog.com website
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Humalog® is a registered trademark of Eli Lilly and Company.
Revised: November 2019
Marketed by: Lilly USA, LLC, Indianapolis, IN 46285, USA
Copyright © 1996, 2019, Eli Lilly and Company. All rights reserved.
LOGVL-0008-IFU-20191125
-
INSTRUCTIONS FOR USE
Instructions for Use
HUMALOG KwikPen®
insulin lispro injection
100 units/mL, 3 mL single-patient-use pen
Read the Instructions for Use before you start taking HUMALOG® and each time you get another KwikPen®. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your HUMALOG KwikPen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
HUMALOG KwikPen (“Pen”) is a disposable single-patient-use prefilled pen containing 300 units of HUMALOG. You can give yourself more than 1 dose from the Pen. Each turn (click) of the Dose Knob dials 1 unit of insulin. You can give from 1 to 60 units in a single injection. If your dose is more than 60 units, you will need to give yourself more than 1 injection. The Plunger only moves a little with each injection, and you may not notice that it moves. The Plunger will only reach the end of the cartridge when you have used all 300 units in the Pen.
People who are blind or have vision problems should not use the Pen without help from a person trained to use the Pen.
How to recognize your HUMALOG KwikPen
- Pen color: Dark blue
- Dose Knob: Dark blue
- Labels: White label with burgundy stripe
Supplies you will need to give your injection
- HUMALOG KwikPen
- KwikPen compatible Needle (Becton, Dickinson and Company Pen Needles recommended)
- Alcohol swab
- Gauze
Preparing your Pen
- Wash your hands with soap and water.
- Check your Pen to make sure you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- Do not use your Pen past the expiration date printed on the Label or for more than 28 days after you first start using the Pen.
- Always use a new needle for each injection to help prevent infections and blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection or get a serious infection from them.
Priming your Pen
Prime before each injection.
- Priming your Pen means removing the air from the Needle and Cartridge that may collect during normal use and ensures that the Pen is working correctly.
- If you do not prime before each injection, you may get too much or too little insulin.
Selecting your dose
- You can give from 1 to 60 units in a single injection.
- If your dose is more than 60 units, you will need to give more than 1 injection.
- - If you need help with dividing up your dose the right way, ask your healthcare provider.
- - Use a new Needle for each injection and repeat the priming step.
- The Pen will not let you dial more than the number of units left in the Pen.
- If you need to inject more than the number of units left in the Pen, you may either:
- - inject the amount left in your Pen and then use a new Pen to give the rest of your dose, or
- - get a new Pen and inject the full dose.
- It is normal to see a small amount of insulin left in the Pen that you can not inject.
Giving your injection
- Inject your insulin as your healthcare provider has shown you.
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Do not try to change your dose while injecting.
After your injection
Step 13:
- Carefully replace the Outer Needle Shield.
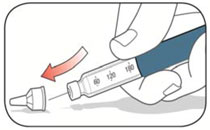
Step 14:
- Unscrew the capped Needle and throw it away (see Disposing of Pens and Needles section).
- Do not store the Pen with the Needle attached to prevent leaking, blocking the Needle, and air from entering the Pen.
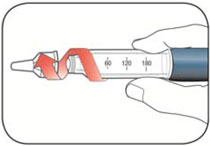
Step 15:
- Replace the Pen Cap by lining up the Cap Clip with the Dose Indicator and pushing straight on.
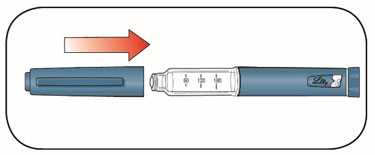
- Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- - made of a heavy-duty plastic,
- - can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- - upright and stable during use,
- - leak-resistant, and
- - properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- The used Pen may be discarded in your household trash after you have removed the needle.
Storing your Pen
Unused Pens
- Store unused Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze your insulin. Do not use if it has been frozen.
- Unused Pens may be used until the expiration date printed on the Label, if the Pen has been kept in the refrigerator.
In-use Pen
- Store the Pen you are currently using at room temperature [up to 86°F (30°C)]. Keep away from heat and light.
- Throw away the HUMALOG Pen you are using after 28 days, even if it still has insulin left in it.
General information about the safe and effective use of your Pen
- Keep your Pen and needles out of the reach of children.
- Do not use your Pen if any part looks broken or damaged.
- Always carry an extra Pen in case yours is lost or damaged.
Troubleshooting
- If you can not remove the Pen Cap, gently twist the cap back and forth, and then pull the cap straight off.
- If the Dose Knob is hard to push:
- - Pushing the Dose Knob more slowly will make it easier to inject.
- - Your Needle may be blocked. Put on a new Needle and prime the Pen.
- - You may have dust, food, or liquid inside the Pen. Throw the Pen away and get a new Pen.
If you have any questions or problems with your HUMALOG KwikPen, contact Lilly at 1-800-LillyRx (1-800-545-5979) or call your healthcare provider for help. For more information on HUMALOG KwikPen and insulin, go to www.humalog.com.
Scan this code to launch
www.humalog.com
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
HUMALOG® and HUMALOG KwikPen® are trademarks of Eli Lilly and Company.
Revised: November 2019
Marketed by: Lilly USA, LLC
Indianapolis, IN 46285, USACopyright © 2007, 2019, Eli Lilly and Company. All rights reserved.
HUMALOG KwikPen meets the current dose accuracy and functional requirements of ISO 11608-1. LOGKP-0007-IFU-20191115
-
INSTRUCTIONS FOR USE
Instructions for Use
HUMALOG® Junior KwikPen®
insulin lispro injection
100 units/mL, 3 mL single-patient-use pen
Read the Instructions for Use before you start taking HUMALOG and each time you get another HUMALOG® Junior KwikPen®. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your HUMALOG Junior KwikPen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
HUMALOG Junior KwikPen (“Pen”) is a disposable single-patient-use prefilled pen containing 300 units of HUMALOG.
- You can give yourself more than 1 dose from the Pen.
- Each turn of the Dose Knob dials 0.5 (½) unit of insulin. You can give from 0.5 (½) to 30 units in a single injection.
- If your dose is more than 30 units, you will need to give yourself more than 1 injection.
- The Plunger only moves a little with each injection, and you may not notice that it moves. The Plunger will only reach the end of the cartridge when you have used all 300 units in the Pen.
People who are blind or have vision problems should not use the Pen without help from a person trained to use the Pen.
How to recognize your HUMALOG Junior KwikPen:
- Pen color:
- Dose Knob:
- Label:
Blue
Blue, with raised ridges on end and side
White with an orange color bar and orange-to-yellow color bandSupplies needed to give your injection:
- HUMALOG Junior KwikPen
- KwikPen compatible Needle (BD [Becton, Dickinson and Company] Pen Needles recommended)
- Alcohol swab
- Gauze
Preparing your Pen
- Wash your hands with soap and water.
- Check the Pen to make sure you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- Do not use your Pen past the expiration date printed on the Label or for more than 28 days after you first start using the Pen.
- Always use a new needle for each injection to help prevent infections and blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection or get a serious infection from them.
Priming your Pen
Prime before each injection.
- Priming your Pen means removing the air from the Needle and Cartridge that may collect during normal use and ensures that the Pen is working correctly.
- If you do not prime before each injection, you may get too much or too little insulin.
Selecting your dose
- You can give from 0.5 (½) to 30 units in a single injection.
- If your dose is more than 30 units, you will need to give more than 1 injection.
- – If you need help with dividing up your dose the right way, ask your healthcare provider.
- – You must use a new Needle for each injection and repeat the priming step.
- The Pen will not let you dial more than the number of units left in the Pen.
- If you need to inject more than the number of units left in the Pen, you may either:
- – inject the amount left in your Pen and then use a new Pen to give the rest of your dose, or
- – get a new Pen and inject the full dose.
- It is normal to see a small amount of insulin left in the Pen that you can not inject.
Giving your injection
- Inject your insulin as your healthcare provider has shown you.
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Do not try to change your dose while injecting.
After your injection
Disposing of Pens and Needles
- Put your used Needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- – made of a heavy-duty plastic,
- – can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- – upright and stable during use,
- – leak-resistant, and
- – properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle the container.
- The used Pen may be discarded in your household trash after you have removed the needle.
Storing your Pen
Unused Pens
- Store unused Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze your insulin. Do not use if it has been frozen.
- Unused Pens may be used until the expiration date printed on the Label, if the Pen has been kept in the refrigerator.
In-use Pen
- Store the Pen you are currently using at room temperature [up to 86°F (30°C)]. Keep away from heat and light.
- Throw away the HUMALOG Pen you are using after 28 days, even if it still has insulin left in it.
General information about the safe and effective use of your Pen
- Keep your Pen and Needles out of the sight and reach of children.
- Do not use your Pen if any part looks broken or damaged.
- Always carry an extra Pen in case yours is lost or damaged.
Troubleshooting
- If you can not remove the Pen Cap, gently twist the cap back and forth, and then pull the cap straight off.
- If the Dose Knob is hard to push:
- – Pushing the Dose Knob more slowly will make it easier to inject.
- – Your Needle may be blocked. Put on a new Needle and prime the Pen.
- – You may have dust, food, or liquid inside the Pen. Throw the Pen away and get a new Pen.
If you have any questions or problems with your HUMALOG Junior KwikPen, contact Lilly at 1-800-LillyRx (1-800-545-5979) or call your healthcare provider for help. For more information on HUMALOG Junior KwikPen and insulin, go to www.humalog.com.
Scan this code to launch
www.humalog.com
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
HUMALOG® and HUMALOG® Junior KwikPen® are trademarks of Eli Lilly and Company.
Revised: November 2019
Marketed by: Lilly USA, LLC
Indianapolis, IN 46285, USA
Copyright © 2017, 2019, Eli Lilly and Company. All rights reserved.
HUMALOG Junior KwikPen meets the current dose accuracy and functional requirements of ISO 11608-1. LOGJRKP-0003-IFU-20191115
-
INSTRUCTIONS FOR USE
Instructions for Use
HUMALOG® Tempo Pen™
insulin lispro injection
100 units/mL, 3 mL pen, single-patient-use
Read the Instructions for Use before you start taking HUMALOG and each time you get another HUMALOG Tempo Pen. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your HUMALOG Tempo Pen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
HUMALOG Tempo Pen (“Pen”) is a disposable single-patient-use prefilled pen containing 300 units of HUMALOG. You can give yourself more than 1 dose from the Pen. Each turn (click) of the Dose Knob dials 1 unit of insulin. You can give from 1 to 60 units in a single injection. If your dose is more than 60 units, you will need to give yourself more than 1 injection. The Plunger only moves a little with each injection, and you may not notice that it moves. The Plunger will only reach the end of the cartridge when you have used all 300 units in the Pen.
People who are blind or have vision problems should not use the Pen without help from a person trained to use the Pen.
This HUMALOG Tempo Pen contains a component that allows for data connectivity when used with a compatible transmitter.
How to recognize your HUMALOG Tempo Pen
- Pen color: Dark blue
- Dose Knob: Burgundy
- Labels: White label with burgundy stripe
Supplies you will need to give your injection
- HUMALOG Tempo Pen
- Tempo Pen compatible Needle (Becton, Dickinson and Company Pen Needles recommended)
- Alcohol swab
- Gauze
Preparing your Pen
- Wash your hands with soap and water.
- Check your Pen to make sure you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- Do not use your Pen past the expiration date printed on the Label or for more than 28 days after you first start using the Pen.
- Always use a new needle for each injection to help prevent infections and blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection or get a serious infection from them.
Priming your Pen
Prime before each injection.
- Priming your Pen means removing the air from the Needle and Cartridge that may collect during normal use and ensures that the Pen is working correctly.
- If you do not prime before each injection, you may get too much or too little insulin.
Selecting your dose
- You can give from 1 to 60 units in a single injection.
- If your dose is more than 60 units, you will need to give more than 1 injection.
- - If you need help with dividing up your dose the right way, ask your healthcare provider.
- - Use a new Needle for each injection and repeat the priming step.
- The Pen will not let you dial more than the number of units left in the Pen.
- If you need to inject more than the number of units left in the Pen, you may either:
- - inject the amount left in your Pen and then use a new Pen to give the rest of your dose, or
- - get a new Pen and inject the full dose.
- It is normal to see a small amount of insulin left in the Pen that you cannot inject.
Giving your injection
- Inject your insulin as your healthcare provider has shown you.
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Do not try to change your dose while injecting.
After your injection
Disposing of Pens and Needles
- Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- - made of a heavy-duty plastic,
- - can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- - upright and stable during use,
- - leak-resistant, and
- - properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- The used Pen may be thrown away (discarded) in your household trash after you have removed the needle.
Storing your Pen
Unopened Pens
- Store unopened Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze your insulin. Do not use if it has been frozen.
- Unopened Pens may be used until the expiration date printed on the Label, if the Pen has been kept in the refrigerator.
In-use Pen
- Store the Pen you are currently using at room temperature [up to 86°F (30°C)]. Keep away from heat and light.
- Throw away the HUMALOG Tempo Pen you are using after 28 days, even if it still has insulin left in it.
General information about the safe and effective use of your Pen
- Keep your Pen and needles out of the reach of children.
- Do not use your Pen if any part looks broken or damaged.
- Always carry an extra Pen in case yours is lost or damaged.
Troubleshooting
- If you cannot remove the Pen Cap, gently twist the cap back and forth, and then pull the cap straight off.
- If the Dose Knob is hard to push:
- - Pushing the Dose Knob more slowly will make it easier to inject.
- - Your Needle may be blocked. Put on a new Needle and prime the Pen.
- - You may have dust, food, or liquid inside the Pen. Throw the Pen away and get a new Pen.
If you have any questions or problems with your HUMALOG Tempo Pen, contact Lilly at 1-800-LillyRx (1-800-545-5979) or call your healthcare provider for help. For more information on HUMALOG Tempo Pen and insulin, go to www.humalog.com.
Scan this code to launch
www.humalog.com
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
HUMALOG® is a registered trademark and TEMPO PENTM is a trademark of Eli Lilly and Company.
Issued: 11/2019
Marketed by: Lilly USA, LLC
Indianapolis, IN 46285, USACopyright © 2019, Eli Lilly and Company. All rights reserved.
HUMALOG Tempo Pen meets the current dose accuracy and functional requirements of ISO 11608-1. LOGTP-0001-IFU-20191115
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration
Revised: November 2019
Patient Information
HUMALOG KwikPen®
insulin lispro injection
U-200 (200 units per mL)Do not share your HUMALOG KwikPen or needles with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
What is HUMALOG?
- HUMALOG is a rapid-acting man-made insulin used to control high blood sugar in adults and children with diabetes mellitus.
- This single-patient-use HUMALOG KwikPen (“Pen”) contains 2 times as much insulin (200U/mL) in 1 mL as standard insulin (100U/mL).
- It is not known if HUMALOG is safe and effective in children less than 3 years of age.
- It is not known if HUMALOG is safe and effective in children with type 2 diabetes.
Who should not take HUMALOG?
Do not take HUMALOG if you:
- are having an episode of low blood sugar (hypoglycemia).
- have an allergy to insulin lispro or any of the ingredients in HUMALOG. See the end of this Patient Information leaflet for a complete list of ingredients in HUMALOG.
What should I tell my healthcare provider before taking HUMALOG?
Before taking HUMALOG, tell your healthcare provider about all your medical conditions, including if you:
- have liver or kidney problems
- take any other medicines, especially ones called TZDs (thiazolidinediones).
- have heart failure or other heart problems. If you have heart failure, it may get worse while you take TZDs with HUMALOG.
- are pregnant or plan to become pregnant. Talk to your healthcare provider about the best way to control your blood sugar if you plan to become pregnant or while you are pregnant.
- are breastfeeding or plan to breastfeed. Talk with your healthcare provider about the best way to feed your baby while taking HUMALOG.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Before you start taking HUMALOG, talk to your healthcare provider about low blood sugar and how to manage it.How should I use HUMALOG KwikPen?
- Read the detailed Instructions for Use that comes with your HUMALOG KwikPen.
- Use HUMALOG KwikPen exactly as your healthcare provider tells you to. Your healthcare provider should tell you how much HUMALOG to take and when to take it.
- Know the type, strength and amount of insulin you take. Do not change the type or amount of insulin you take unless your healthcare provider tells you to. The amount of insulin and the best time for you to take your insulin may need to change if you take different types of insulin.
- Check your insulin label each time you give your injection to make sure you are taking the correct insulin.
- HUMALOG comes in a KwikPen which is a disposable single-patient use prefilled pen that you must use to give your HUMALOG. The dose window on your pen shows your dose of HUMALOG. Do not make any dose changes unless your healthcare provider tells you to.
- Do not use a syringe to remove HUMALOG from your KwikPen disposable prefilled pen.
- Do not re-use needles. Always use a new needle for each injection. Re-use of needles increases your risk of having blocked needles, which may cause you to get the wrong dose of HUMALOG. Using a new needle for each injection also lowers your risk of getting an infection. If your needle is blocked, follow the instructions in the “General information about the safe and effective use of your Pen” section of the Instructions for Use.
- HUMALOG is a rapid-acting insulin. Inject HUMALOG within 15 minutes before or right after eating a meal.
- Inject HUMALOG under the skin (subcutaneously) of your stomach area, buttocks, upper legs or upper arms. Do not use HUMALOG KwikPen (“Pen”) in an insulin pump or inject HUMALOG KwikPen into your vein (intravenously).
-
Change (rotate) your injection sites within the area you choose with each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not use the exact same spot for each injection.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Do not mix the HUMALOG in the HUMALOG KwikPen with any other type of insulin or liquid medicine.
- Check your blood sugar levels. Ask your healthcare provider what your blood sugar should be and when you should check your blood sugar levels.
Keep HUMALOG KwikPen and all medicines out of reach of children. Your dose of HUMALOG may need to change because of a:
- change in physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, or because of other medicines you take.
What should I avoid while using HUMALOG KwikPen?
While using HUMALOG KwikPen do not:
- drive or operate heavy machinery, until you know how HUMALOG KwikPen affects you
- drink alcohol or take prescription or over-the-counter medicines that contain alcohol
What are the possible side effects of HUMALOG?
HUMALOG may cause serious side effects that can lead to death, including:
-
low blood sugar (hypoglycemia). Signs and symptoms of low blood sugar may include:
- dizziness or light-headedness
- sweating
- confusion
- headache
- blurred vision
- slurred speech
- shakiness
- fast heartbeat
- anxiety, irritability or mood changes
- hunger
Your healthcare provider may prescribe a glucagon emergency kit so that someone else can give you glucagon if your blood sugar becomes too low (severe hypoglycemia) and you are unable to take sugar by mouth. - severe allergic reaction (whole body reaction). Get medical help right away, if you have any of these signs or symptoms of a severe allergic reaction:
- a rash over your whole body
- trouble breathing
- a fast heartbeat
- sweating
- low potassium in your blood (hypokalemia).
- heart failure. Taking certain diabetes pills called thiazolidinediones or “TZDs” with HUMALOG may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with HUMALOG. Your healthcare provider should monitor you closely while you are taking TZDs with HUMALOG. Tell your healthcare provider if you have any new or worse symptoms of heart failure including:
- shortness of breath
- swelling of your ankles or feet
- sudden weight gain
Treatment with TZDs and HUMALOG may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
Get emergency medical help if you have:
- trouble breathing
- shortness of breath
- fast heartbeat
- swelling of your face, tongue, or throat
- sweating
- extreme drowsiness
- dizziness
- confusion
The most common side effects of HUMALOG include: - low blood sugar (hypoglycemia)
- reactions at your injection site
- skin thickening or pits at the injection site (lipodystrophy)
- weight gain
- swelling in your hands or feet
- itching
- rash
These are not all of the possible side effects of HUMALOG. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. General Information about the safe and effective use of HUMALOG KwikPen.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not take HUMALOG for a condition for which it was not prescribed. Do not give HUMALOG to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about HUMALOG KwikPen. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about HUMALOG that is written for healthcare professionals.What are the ingredients in HUMALOG U-200?
Active ingredient: insulin lispro.
Inactive ingredient: glycerin, tromethamine, metacresol, zinc oxide (zinc ion), trace amounts of phenol and water for injection.
Humalog® and Humalog KwikPen® are registered trademarks of Eli Lilly and Company.
Marketed by: Lilly USA, LLC, Indianapolis, IN 46285, USA
Copyright © 2015, 2019, Eli Lilly and Company. All rights reserved.
For more information, go to www.humalog.com or call 1-800-545-5979.LOG200-0003-PPI-20191115
-
INSTRUCTIONS FOR USE
Instructions for Use
HUMALOG KwikPen®
insulin lispro injection
200 units/mL, 3 mL single-patient-use pen
Read the Instructions for Use before you start taking HUMALOG® and each time you get another KwikPen®. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your HUMALOG KwikPen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
HUMALOG KwikPen 200 units/mL (“Pen”) is a disposable single-patient-use prefilled pen containing 600 units of HUMALOG. You can give yourself more than 1 dose from the Pen. Each turn (click) of the Dose Knob dials 1 unit of insulin. You can give from 1 to 60 units in a single injection. If your dose is more than 60 units, you will need to give yourself more than 1 injection. The Plunger only moves a little with each injection, and you may not notice that it moves. The Plunger will only reach the end of the cartridge when you have used all 600 units in the Pen.
HUMALOG KwikPen is available in 2 strengths, 100 units/mL and 200 units/mL. Inject HUMALOG 200 units/mL only with your Pen. Do not transfer insulin from your Pen to a syringe. Syringes will not measure 200 units/mL insulin correctly. A severe overdose can result, causing very low blood sugar which may put your life in danger.
People who are blind or have vision problems should not use the Pen without help from a person trained to use the Pen.
How to recognize your HUMALOG 200 units/mL KwikPen
- Pen color: Dark grey
- Dose Knob: Dark grey with burgundy ring on the end
- Label: Burgundy label with “200 units per mL (U-200)” in white stripe and a grey and burgundy checker board design.
Supplies needed to give your injection
- HUMALOG KwikPen
- KwikPen compatible Needle (Becton, Dickinson and Company Pen Needles recommended)
- Alcohol swab
- Gauze
Preparing your Pen
- Wash your hands with soap and water.
- Check your Pen to make sure you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- Do not use your Pen past the expiration date printed on the Label or for more than 28 days after you first start using the Pen.
- Always use a new needle for each injection to help prevent infections and blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection or get a serious infection from them.
Priming your Pen
Prime before each injection.
- Priming your Pen means removing the air from the Needle and Cartridge that may collect during normal use and ensures that the Pen is working correctly.
- If you do not prime before each injection, you may get too much or too little insulin.
Step 6:
- To prime your Pen, turn the Dose Knob to select 2 units.
Step 7:
- Hold your Pen with the Needle pointing up. Tap the Cartridge Holder gently to collect air bubbles at the top.
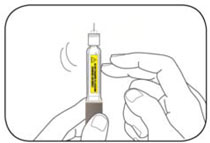
Selecting your dose
This Pen has been made to deliver the dose that is shown in the Dose Window. Dial your usual dose as instructed by your healthcare provider.
- You can give from 1 to 60 units in a single injection.
- If your dose is more than 60 units, you will need to give more than 1 injection.
- - If you need help with dividing up your dose the right way, ask your healthcare provider.
- - Use a new Needle for each injection and repeat the priming step.
- The Pen will not let you dial more than the number of units left in the Pen.
- If you need to inject more than the number of units left in the Pen, you may either:
- -
inject the amount left in your Pen and then use a new Pen to give the rest of your dose,
or - - get a new Pen and inject the full dose.
- It is normal to see a small amount of insulin left in the Pen that you can not inject. Do not transfer this to a syringe. Severe overdose can result.
Giving your injection
- Inject your insulin as your healthcare provider has shown you.
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not use the exact same spot for each injection.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Do not try to change your dose while injecting.
After your injection
Disposing of Pens and Needles
- Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- - made of a heavy-duty plastic,
- - can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- - upright and stable during use,
- - leak-resistant, and
- - properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- The used Pen may be discarded in your household trash after you have removed the needle.
Storing your Pen
Unused Pens
- Store unused Pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze your insulin. Do not use if it has been frozen.
- Unused Pens may be used until the expiration date printed on the Label, if the Pen has been kept in the refrigerator.
In-use Pen
- Store the Pen you are currently using at room temperature [up to 86°F (30°C)]. Keep away from heat and light.
- Throw away the HUMALOG Pen you are using after 28 days, even if it still has insulin left in it.
General information about the safe and effective use of your Pen
- Keep your Pen and needles out of the sight and reach of children.
- Do not use your Pen if any part looks broken or damaged.
- Always carry an extra Pen in case yours is lost or damaged.
Troubleshooting
- If you can not remove the Pen Cap, gently twist the cap back and forth, and then pull the cap straight off.
- If the Dose Knob is hard to push:
- - Pushing the Dose Knob more slowly will make it easier to inject.
- - Your Needle may be blocked. Put on a new Needle and prime the Pen.
- - You may have dust, food, or liquid inside the Pen. Throw the Pen away and get a new Pen.
If you have any questions or problems with your HUMALOG KwikPen, contact Lilly at 1-800-LillyRx (1-800-545-5979) or call your healthcare provider for help. For more information on HUMALOG KwikPen and insulin, go to www.humalog.com.
Scan this code to launch
www.humalog.com
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
HUMALOG® and HUMALOG KwikPen® are trademarks of Eli Lilly and Company.
Revised: November 2019
Marketed by: Lilly USA, LLC
Indianapolis, IN 46285, USACopyright © 2015, 2019, Eli Lilly and Company. All rights reserved.
HUMALOG KwikPen meets the current dose accuracy and functional requirements of ISO 11608-1. LOGKP200-0004-IFU-20191115
-
PRINCIPAL DISPLAY PANEL
PACKAGE CARTON – HUMALOG 10 mL vial
NDC: 0002-7510-01
10 mL
100 units per mL
Humalog®
insulin lispro injection
Rx only
U-100
www.humalog.com
Lilly
-
PRINCIPAL DISPLAY PANEL
PACKAGE CARTON – HUMALOG KwikPen
NDC: 0002-8799-59
Humalog KwikPen®
insulin lispro injection
For Single Patient Use Only
Dispense in this sealed carton.
U-100
100 units per mL
Needles not included
This device is recommended for use with Becton, Dickinson and Company's insulin pen needles
prefilled insulin delivery device
Rx only
5 x 3 mL Prefilled Pens
Read Insulin Delivery Device Instructions for Use
-
PRINCIPAL DISPLAY PANEL
PACKAGE CARTON – HUMALOG Cartridge 3mL
NDC: 0002-7516-59
100 units per mL
VL-7516
5 x 3 mL Cartridges
Humalog®
insulin lispro injection
For Single Patient Use Only
Rx only
U-100
For use in Eli Lilly and Company's HumaPen® LUXURA® HD insulin delivery device.
www.humalog.com
Lilly
-
PRINCIPAL DISPLAY PANEL
PACKAGE CARTON – HUMALOG U200 KwikPen 2ct
Dispense in this sealed carton.
NDC: 0002-7712-27
Humalog KwikPen®
insulin lispro injection
For Single Patient Use Only
200 units per mL (U-200)
prefilled insulin delivery device
2 x 3 mL Prefilled Pens
Rx only
For subcutaneous use.
Read Insulin Delivery Device Instructions for Use.
NEEDLES NOT INCLUDED
This device is recommended for use with Becton, Dickinson and Company's insulin pen needles.
-
PRINCIPAL DISPLAY PANEL
PACKAGE CARTON – HUMALOG Junior KwikPen
Dispense in this sealed carton.
NDC: 0002-7714-59
Humalog®
Junior KwikPen®
insulin lispro injection
For Single Patient Use Only
100 units per mL (U-100)
The pen can deliver 0.5 (½) to 30 units in one injection.
5 x 3 mL prefilled pens
prefilled insulin delivery device
Rx only
Read Humalog® Junior KwikPen® Instructions for Use.
NEEDLES NOT INCLUDED
This device is recommended for use with Becton, Dickinson and Company's insulin pen needles.
Lilly
-
PRINCIPAL DISPLAY PANEL
PACKAGE CARTON – HUMALOG Tempo Pen
NDC: 0002-8213-05
Humalog®
Tempo PenTM
insulin lispro injection
For Single Patient Use Only
HP-8213
Dispense in this sealed carton.
U-100
100 units per mL
Needles not included
This device is recommended for use with Becton, Dickinson and Company's insulin pen needles
For subcutaneous use only.
prefilled insulin delivery device
Rx only
5 x 3 mL Prefilled Pens
Read Insulin Delivery Device Instructions for Use
-
INGREDIENTS AND APPEARANCE
HUMALOG
insulin lispro injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-7510 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Insulin lispro (UNII: GFX7QIS1II) (Insulin lispro - UNII:GFX7QIS1II) Insulin lispro 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) 16 mg in 1 mL Sodium Phosphate, Dibasic (UNII: GR686LBA74) 1.88 mg in 1 mL Metacresol (UNII: GGO4Y809LO) 3.15 mg in 1 mL Zinc (UNII: J41CSQ7QDS) .0197 mg in 1 mL Phenol (UNII: 339NCG44TV) Water (UNII: 059QF0KO0R) Hydrochloric acid (UNII: QTT17582CB) Sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-7510-01 1 in 1 CARTON 07/24/1996 1 10 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 0002-7510-17 1 in 1 CARTON 12/04/2009 2 3 mL in 1 VIAL; Type 0: Not a Combination Product 3 NDC: 0002-7510-99 1 in 1 CARTON 07/24/1996 3 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020563 06/14/1996 HUMALOG KWIKPEN
insulin lispro injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-8799 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Insulin lispro (UNII: GFX7QIS1II) (Insulin lispro - UNII:GFX7QIS1II) Insulin lispro 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) 16 mg in 1 mL Sodium Phosphate, Dibasic (UNII: GR686LBA74) 1.88 mg in 1 mL Metacresol (UNII: GGO4Y809LO) 3.15 mg in 1 mL Zinc (UNII: J41CSQ7QDS) .0197 mg in 1 mL Phenol (UNII: 339NCG44TV) Water (UNII: 059QF0KO0R) Hydrochloric acid (UNII: QTT17582CB) Sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-8799-59 5 in 1 CARTON 01/16/2008 1 NDC: 0002-8799-01 3 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 0002-8799-99 1 in 1 CARTON 01/16/2008 2 3 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC: 0002-8799-61 1 in 1 CARTON 10/01/2017 3 3 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020563 09/06/2007 HUMALOG
insulin lispro injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-7516 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Insulin lispro (UNII: GFX7QIS1II) (Insulin lispro - UNII:GFX7QIS1II) Insulin lispro 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) 16 mg in 1 mL Sodium Phosphate, Dibasic (UNII: GR686LBA74) 1.88 mg in 1 mL Metacresol (UNII: GGO4Y809LO) 3.15 mg in 1 mL Zinc (UNII: J41CSQ7QDS) .0197 mg in 1 mL Phenol (UNII: 339NCG44TV) Water (UNII: 059QF0KO0R) Hydrochloric acid (UNII: QTT17582CB) Sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-7516-59 5 in 1 CARTON 07/26/2000 1 NDC: 0002-7516-01 3 mL in 1 CARTRIDGE; Type 0: Not a Combination Product 2 NDC: 0002-7516-99 5 in 1 CARTON 07/26/2000 2 3 mL in 1 CARTRIDGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020563 02/20/1998 HUMALOG KWIKPEN
insulin lispro injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-7712 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Insulin lispro (UNII: GFX7QIS1II) (Insulin lispro - UNII:GFX7QIS1II) Insulin lispro 200 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) 16 mg in 1 mL Tromethamine (UNII: 023C2WHX2V) 5 mg in 1 mL Metacresol (UNII: GGO4Y809LO) 3.15 mg in 1 mL Zinc (UNII: J41CSQ7QDS) .046 mg in 1 mL Phenol (UNII: 339NCG44TV) Water (UNII: 059QF0KO0R) Hydrochloric acid (UNII: QTT17582CB) Sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-7712-27 2 in 1 CARTON 05/26/2015 1 NDC: 0002-7712-01 3 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 0002-7712-99 1 in 1 CARTON 05/26/2015 2 3 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC: 0002-7712-61 1 in 1 CARTON 10/01/2017 3 3 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205747 05/26/2015 HUMALOG JUNIOR KWIKPEN
insulin lispro injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-7714 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Insulin lispro (UNII: GFX7QIS1II) (Insulin lispro - UNII:GFX7QIS1II) Insulin lispro 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) 16 mg in 1 mL Sodium Phosphate, Dibasic (UNII: GR686LBA74) 1.88 mg in 1 mL Metacresol (UNII: GGO4Y809LO) 3.15 mg in 1 mL Zinc (UNII: J41CSQ7QDS) .0197 mg in 1 mL Phenol (UNII: 339NCG44TV) Water (UNII: 059QF0KO0R) Hydrochloric acid (UNII: QTT17582CB) Sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-7714-59 5 in 1 CARTON 09/14/2017 1 NDC: 0002-7714-01 3 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 0002-7714-61 1 in 1 CARTON 09/25/2017 2 3 mL in 1 SYRINGE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020563 06/06/2017 HUMALOG TEMPO PEN
insulin lispro injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0002-8213 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Insulin lispro (UNII: GFX7QIS1II) (Insulin lispro - UNII:GFX7QIS1II) Insulin lispro 100 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) 16 mg in 1 mL Sodium Phosphate, Dibasic (UNII: GR686LBA74) 1.88 mg in 1 mL Metacresol (UNII: GGO4Y809LO) 3.15 mg in 1 mL Zinc (UNII: J41CSQ7QDS) .0197 mg in 1 mL Phenol (UNII: 339NCG44TV) Water (UNII: 059QF0KO0R) Hydrochloric acid (UNII: QTT17582CB) Sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0002-8213-05 5 in 1 CARTON 11/15/2019 1 NDC: 0002-8213-01 3 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020563 06/06/2017 Labeler - Eli Lilly and Company (006421325)
Trademark Results [Humalog]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HUMALOG 74314093 1963572 Live/Registered |
Eli Lilly and Company 1992-09-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
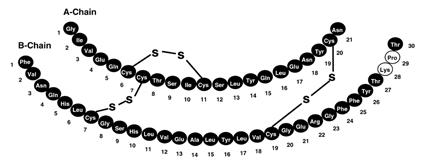

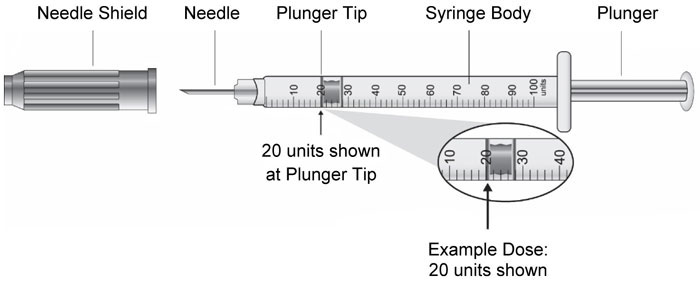
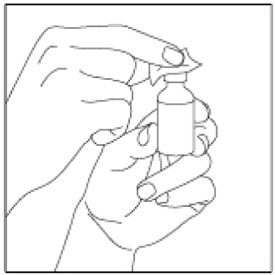
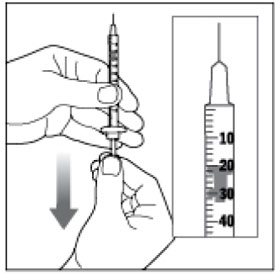 (Example Dose: 20 units shown)
(Example Dose: 20 units shown)
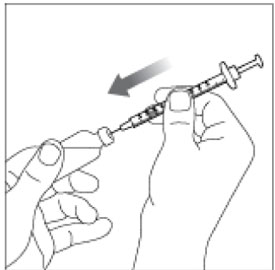
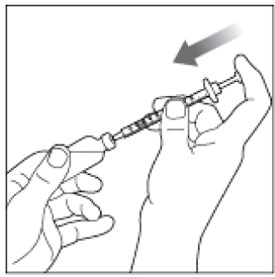
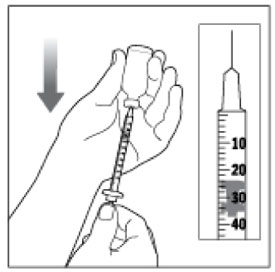
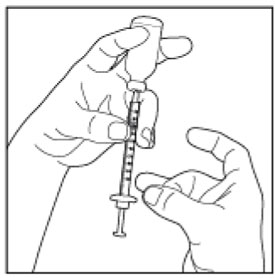
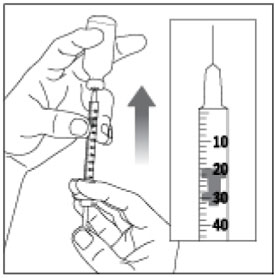 (Example Dose: 20 units shown)
(Example Dose: 20 units shown)
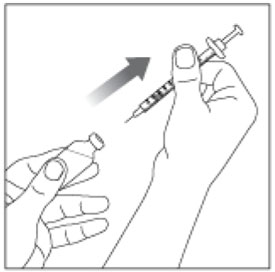
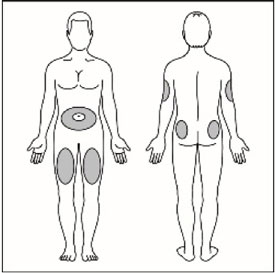
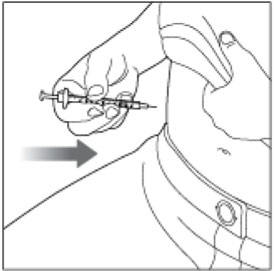
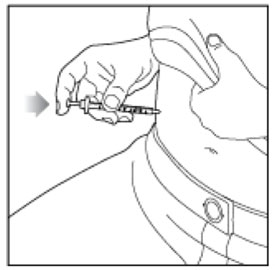
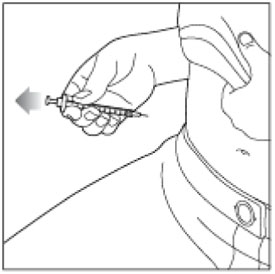


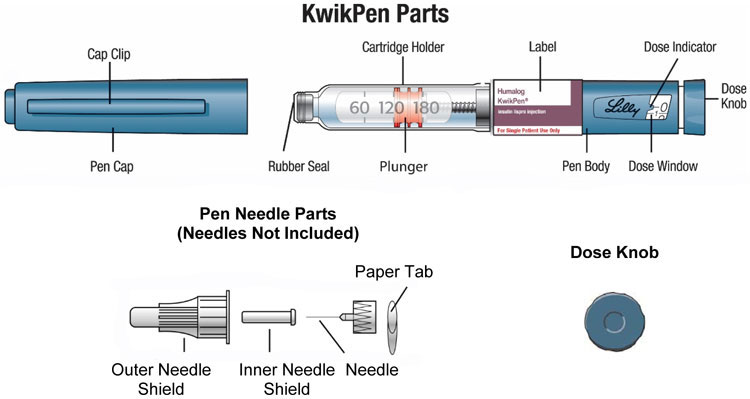
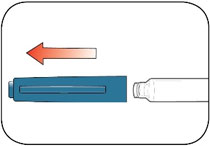
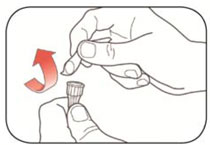
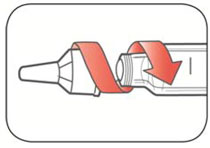
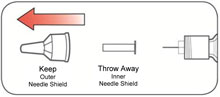
 (Example: 12 units shown in the Dose Window)
(Example: 12 units shown in the Dose Window) (Example: 25 units shown in the Dose Window)
(Example: 25 units shown in the Dose Window)
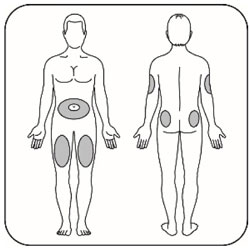
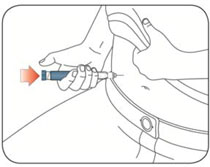


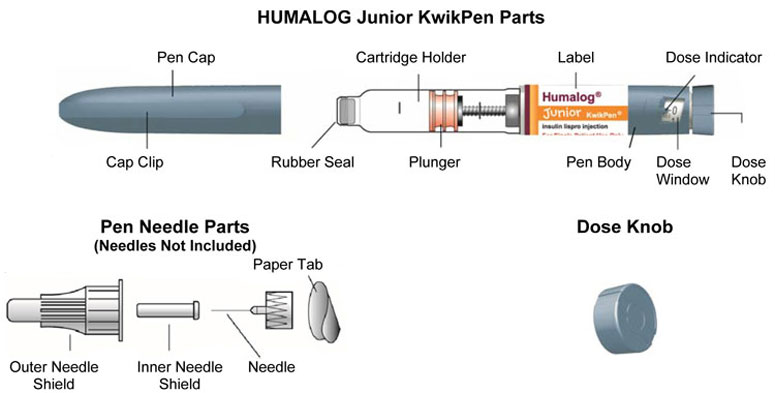
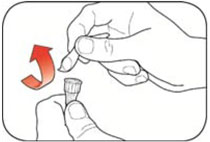
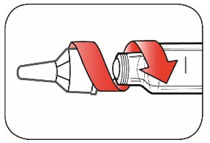
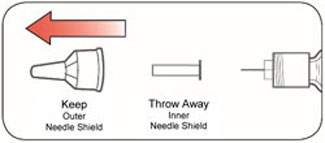
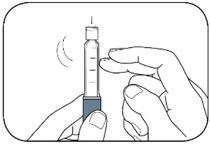
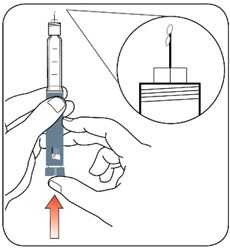
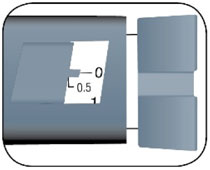
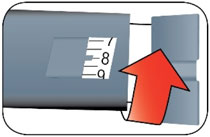
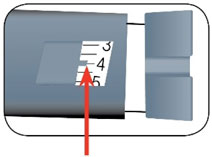 Example: 4 units shown in the Dose Window
Example: 4 units shown in the Dose Window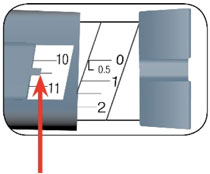
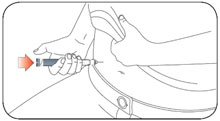
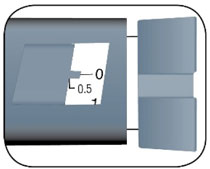
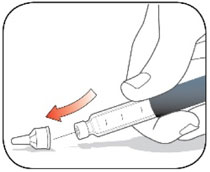
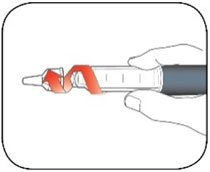

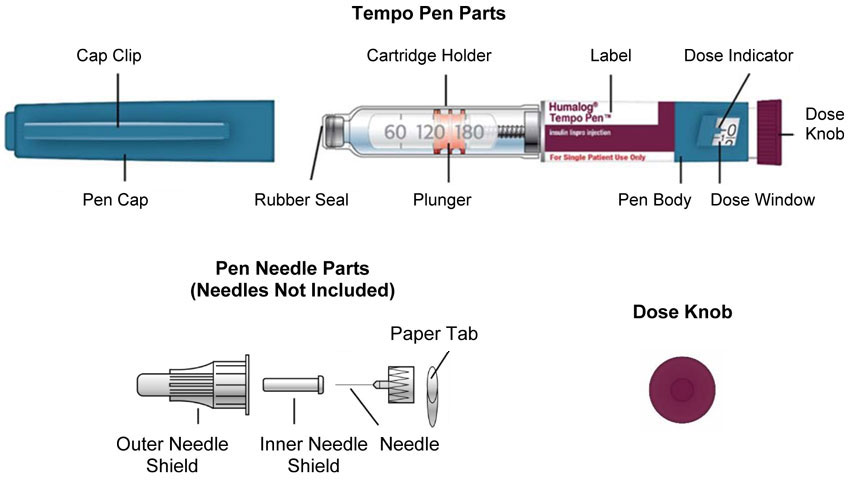
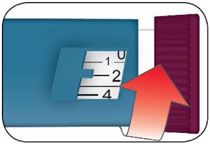
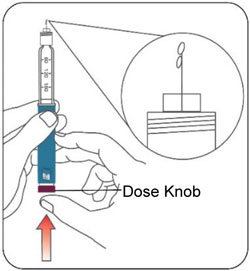
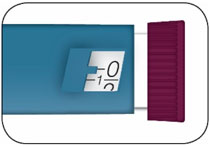
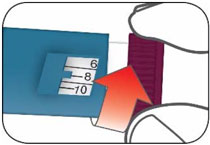
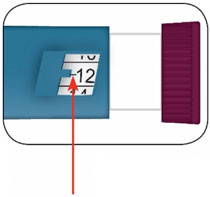 (Example: 12 units shown in the Dose Window)
(Example: 12 units shown in the Dose Window)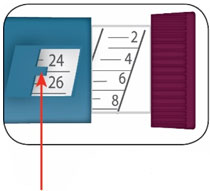 (Example: 25 units shown in the Dose Window)
(Example: 25 units shown in the Dose Window)
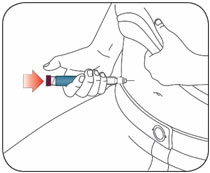

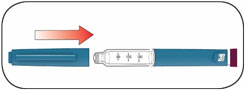


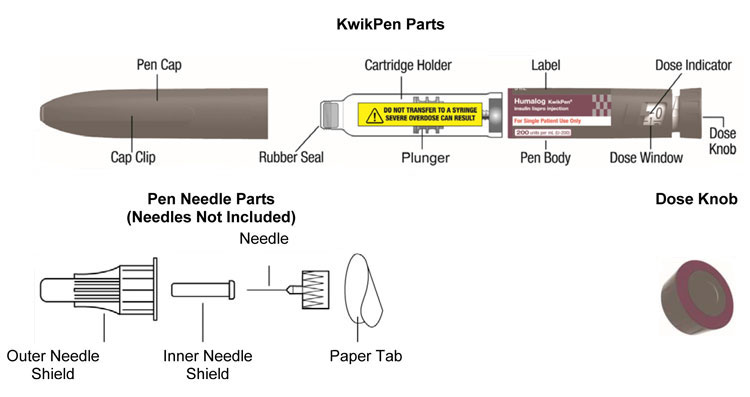
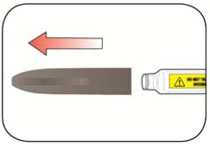
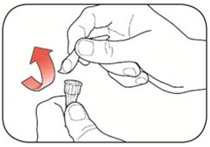
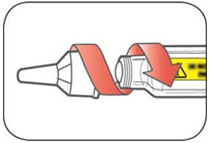
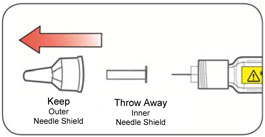
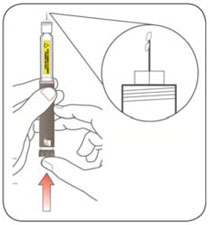
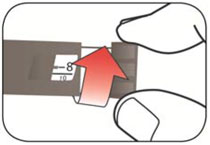
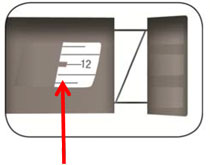 (Example: 12 units shown in the Dose Window)
(Example: 12 units shown in the Dose Window)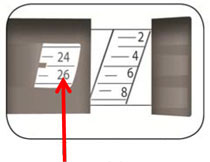 (Example: 25 units shown in the Dose Window)
(Example: 25 units shown in the Dose Window)
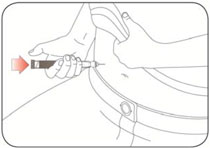
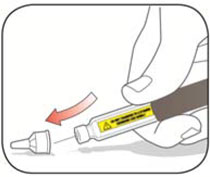
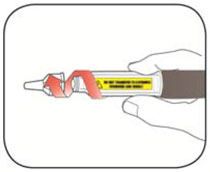
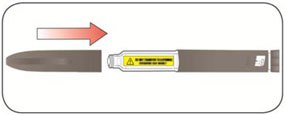
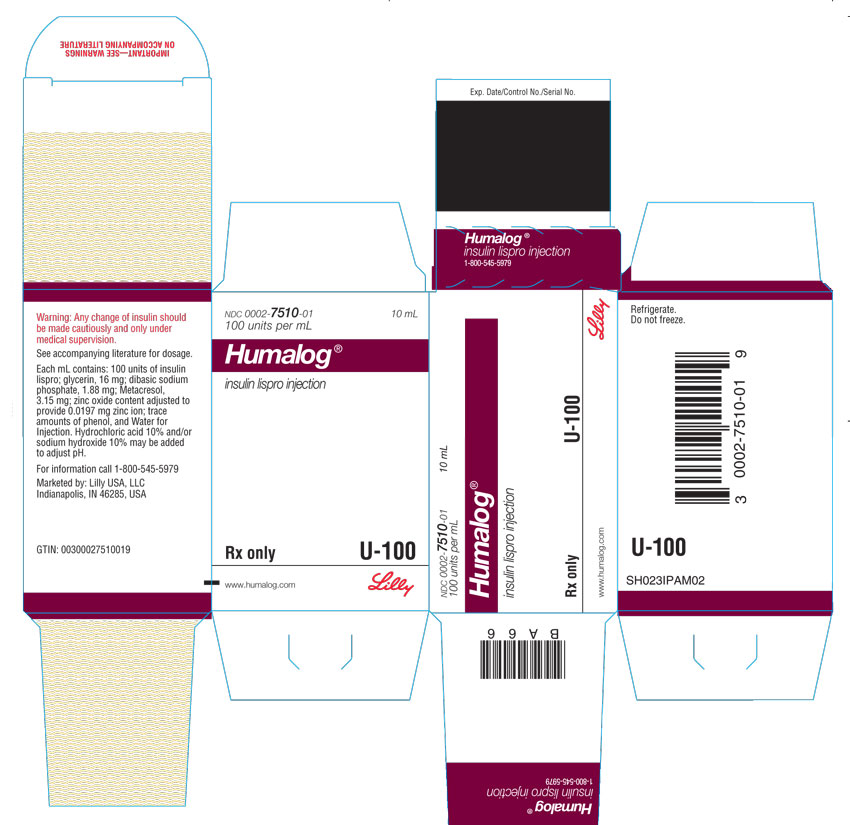
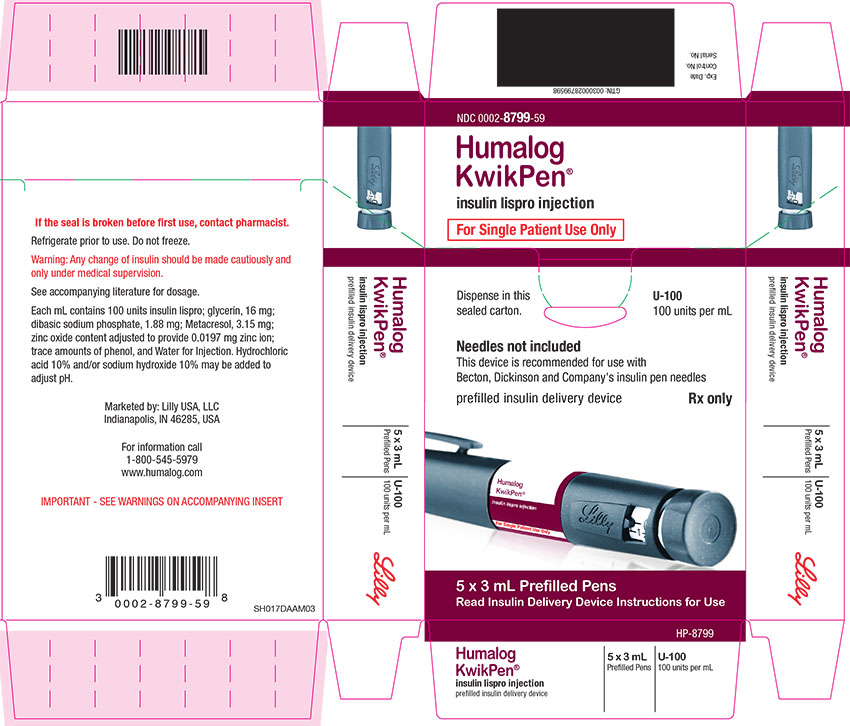
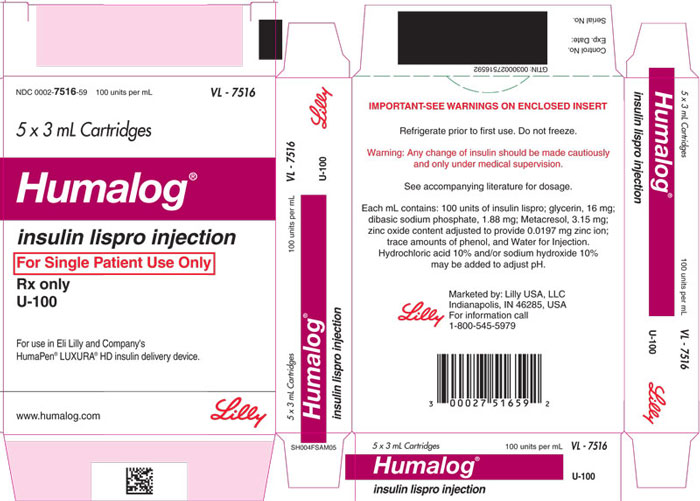
 DO NOT TRANSFER TO A SYRINGE SEVERE OVERDOSE CAN RESULT
DO NOT TRANSFER TO A SYRINGE SEVERE OVERDOSE CAN RESULT