ENFLONSIA- clesrovimab injection, solution
ENFLONSIA by
Drug Labeling and Warnings
ENFLONSIA by is a Prescription medication manufactured, distributed, or labeled by Merck Sharp & Dohme LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ENFLONSIA safely and effectively. See full prescribing information for ENFLONSIA.
ENFLONSIA™ (clesrovimab-cfor) injection, for intramuscular use
Initial U.S. Approval: 2025INDICATIONS AND USAGE
ENFLONSIA is a respiratory syncytial virus (RSV) F protein-directed fusion inhibitor indicated for the prevention of RSV lower respiratory tract disease in neonates and infants who are born during or entering their first RSV season. (1)
DOSAGE AND ADMINISTRATION
Recommended dosage: 105 mg administered as a single intramuscular (IM) injection. (2.1)
DOSAGE FORMS AND STRENGTHS
Injection: 105 mg/0.7 mL in a single-dose prefilled syringe. (3)
CONTRAINDICATIONS
ENFLONSIA is contraindicated in infants with a history of serious hypersensitivity reactions, including anaphylaxis, to any component of ENFLONSIA. (4)
WARNINGS AND PRECAUTIONS
Hypersensitivity Including Anaphylaxis: Serious hypersensitivity reactions, including anaphylaxis, have been observed with other human immunoglobulin G1 (IgG1) monoclonal antibodies. Initiate appropriate medications and/or supportive care if such reactions occur. (5.1)
ADVERSE REACTIONS
Most common adverse reactions were injection-site erythema (3.8%), injection-site swelling (2.7%) and rash (2.3%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
The safety and effectiveness of ENFLONSIA have not been established in children older than 12 months of age. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Including Anaphylaxis
5.2 RSV Diagnostic Test Interference
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Interference with Rapid Antigen Detection RSV Diagnostic Assays
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Description of Clinical Trials
14.2 Prevention of RSV-Associated Disease in Neonates and Infants (≥29 Weeks GA) Entering Their First RSV Season (Trial 004)
14.3 Prevention of RSV-Associated Disease in Infants Born at ≤35 Weeks Gestational Age and Infants with CLD of Prematurity or Hemodynamically Significant CHD Entering Their First RSV Season (Trial 007)
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose for neonates and infants born during or entering their first RSV season is 105 mg administered as a single intramuscular (IM) injection.
For neonates and infants born during the RSV season, administer ENFLONSIA once starting from birth. For infants born outside the RSV season, administer ENFLONSIA once prior to the start of their first RSV season considering the duration of protection provided by ENFLONSIA [see Clinical Pharmacology (12.2)].
Infants Undergoing Cardiac Surgery with Cardiopulmonary Bypass
For infants undergoing cardiac surgery with cardiopulmonary bypass during or entering their first RSV season, an additional 105 mg dose administered as an IM injection is recommended as soon as the infant is stable after surgery to ensure adequate clesrovimab-cfor serum levels.
2.2 Administration Instructions
ENFLONSIA must be administered by a healthcare provider.
Before injection, remove ENFLONSIA from the refrigerator and allow the prefilled syringe to come to room temperature for approximately 15 minutes. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. ENFLONSIA is a clear to slightly opalescent, colorless to slightly yellow solution. This product should not be used if particulate matter or discoloration is found. Do not use if the prefilled syringe has been dropped or damaged, the security seal on the carton has been broken, or the expiration date has passed. Refer to Figure 1 for prefilled syringe components.
Figure 1: Prefilled Syringe Components 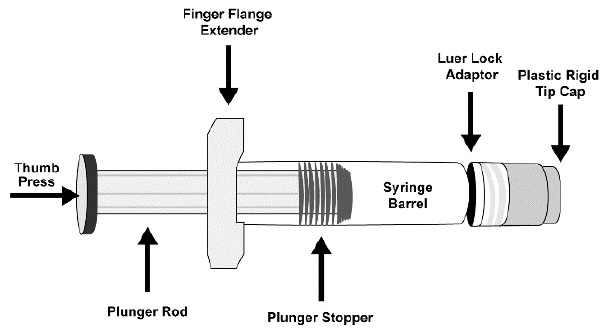
Step 1: Hold the syringe barrel in one hand and unscrew the tip cap by twisting it counter-clockwise with the other hand. Do not remove the Luer Lock adaptor and the finger flange extender.
Step 2: Attach a sterile Luer Lock needle by twisting in a clockwise direction until the needle fits securely on the syringe. Due to the viscosity of the product, use a 22- to 25-gauge needle.
Step 3: Inject the entire contents of the ENFLONSIA prefilled syringe intramuscularly in the anterolateral aspect of the thigh. ENFLONSIA should not be injected in the gluteal area or areas where there may be a major nerve trunk and/or blood vessel.
Step 4: Discard syringe into a sharps container.
Co-administration with Childhood Vaccines and Immunoglobulin Products
ENFLONSIA can be given concomitantly with childhood vaccines [see Clinical Pharmacology (12.3)]. When ENFLONSIA is administered concomitantly with injectable vaccines, it should be given using a separate syringe and at a different injection site. Do not mix ENFLONSIA with any vaccines or medications in the same syringe or vial.There is no information regarding co-administration of ENFLONSIA with other immunoglobulin products. There are no data regarding substitution of ENFLONSIA for palivizumab once prophylaxis treatment is initiated with palivizumab for the RSV season.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
ENFLONSIA is contraindicated in infants with a history of serious hypersensitivity reactions, including anaphylaxis, to any component of ENFLONSIA [see Warnings and Precautions (5.1) and Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Including Anaphylaxis
Serious hypersensitivity reactions, including anaphylaxis, have been observed with other human immunoglobulin G1 (IgG1) monoclonal antibodies. If signs or symptoms of a clinically significant hypersensitivity reaction or anaphylaxis occur, initiate appropriate medications and/or supportive therapy.
5.2 RSV Diagnostic Test Interference
Clesrovimab-cfor may interfere with some immunologically-based RSV diagnostic assays (i.e., rapid antigen tests) as observed in laboratory studies. Confirmation using a reverse transcriptase polymerase chain reaction (RT-PCR) assay is recommended when rapid antigen assay results are negative and clinical observations are consistent with RSV infection [see Drug Interactions (7.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ENFLONSIA was evaluated in 2,858 infants who received ENFLONSIA in Phase 2b/3 and Phase 3 clinical trials (Trial 004 and Trial 007).
Neonates and Infants Entering Their First RSV Season (Trial 004)
Trial 004 was a Phase 2b/3, randomized, double-blind placebo-controlled, multisite trial conducted in early and moderate preterm infants (≥29 to <35 weeks gestational age (GA)) and late preterm and full-term infants (≥35 weeks GA). Participants were randomized 2:1 and received a single 105 mg dose of ENFLONSIA (N=2,412, including 422 early and moderate preterm infants) or saline placebo (N=1,202, including 209 early and moderate preterm infants) by IM injection. Participants were monitored for 30 minutes post-dose. Safety was assessed using an electronic diary device from Days 1 through 42 post-dose. Participants were monitored for serious adverse events (SAEs) through the duration of their participation for up to 365 days post-dose. A subset of participants was monitored for SAEs for up to 515 days post-dose.
Table 1 summarizes the adverse reactions in participants who received ENFLONSIA. Most (≥97%) of the adverse reactions were toxicity grade 1 (mild) or grade 2 (moderate).
Table 1: Adverse Reactions Reported at an Incidence Higher Than Placebo (Trial 004) Adverse Reaction ENFLONSIA
N=2,412*
%Placebo
N=1,202*
%- * Sample size reflects the number of participants included in the safety analysis population.
- † Solicited on Day 1 through Day 5 post-dose using an electronic diary device.
- ‡ Defined by the following grouped preferred terms: rash, rash erythematous, rash macular, rash papular, rash maculo-papular, rash vesicular, rash exfoliative, dermatitis allergic, drug eruption and toxic skin eruption.
Injection-site erythema† (occurring within 5 days post-dose) 3.8 3.3 Injection-site swelling† (occurring within 5 days post-dose) 2.7 2.6 Rash‡ (occurring within 14 days post-dose) 2.3 1.9 Infants Born at ≤35 Weeks Gestational Age and Infants with Chronic Lung Disease (CLD) of Prematurity or Hemodynamically Significant Congenital Heart Disease (CHD) Entering Their First RSV Season (Trial 007)
Trial 007 was a Phase 3, randomized, partially-blind, palivizumab-controlled, multisite trial conducted in infants at increased risk of severe RSV disease. Participants were randomized and received a single 105 mg dose of ENFLONSIA (N=446) followed by a dose of placebo one month later or 3 to 5 monthly doses of 15 mg/kg palivizumab (N=450) by IM injection. Of the 446 participants who received ENFLONSIA, 176 had CLD of prematurity or CHD and 270 were early or moderate preterm infants (≤35 weeks GA) without CLD of prematurity or CHD. Participants were monitored for 30 minutes post-dose. Safety was assessed using an electronic diary device from Day 1 (dose 1) through 14 days post-dose 2, and 14 days after each subsequent dose. Participants were monitored for serious adverse events in the first RSV season for up to 365 days.
The safety profile of ENFLONSIA in infants at increased risk of severe RSV disease entering their first season was similar to palivizumab and consistent with the safety profile of ENFLONSIA in infants in Trial 004.
-
7 DRUG INTERACTIONS
7.1 Interference with Rapid Antigen Detection RSV Diagnostic Assays
Clesrovimab-cfor may interfere with some immunologically-based RSV diagnostic assays (i.e., rapid antigen tests) as observed in laboratory studies. Confirmation using an RT-PCR assay is recommended when rapid antigen RSV diagnostic assay results are negative and clinical observations are consistent with RSV infection. Clesrovimab-cfor does not interfere with RT-PCR diagnostic assays [see Warnings and Precautions (5.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
The safety and effectiveness of ENFLONSIA have been established for the prevention of RSV lower respiratory tract disease in neonates and infants born during or entering their first RSV season and the information on this use is discussed throughout the labeling.
The safety and effectiveness of ENFLONSIA have not been established in children older than 12 months of age.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Clesrovimab-cfor is a respiratory syncytial virus F protein-directed fusion inhibitor. Clesrovimab-cfor is a fully human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody produced in recombinant Chinese hamster ovary (CHO) cells. The molecular weight is approximately 149 kDa.
ENFLONSIA (clesrovimab-cfor) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution for intramuscular injection.
Each 0.7 mL contains 105 mg of clesrovimab-cfor, arginine hydrochloride (10.33 mg), histidine (0.55 mg), L-histidine monohydrochloride monohydrate (0.74 mg), polysorbate 80 (0.14 mg), sucrose (35 mg) and water for injection (USP). The pH is 6.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
ENFLONSIA is a monoclonal antibody with anti-RSV activity [see Microbiology (12.4)].
12.2 Pharmacodynamics
RSV serum neutralizing antibody titer correlates with clesrovimab-cfor serum concentration. Following IM administration of clesrovimab-cfor in infants, the RSV neutralizing antibody titers in serum were estimated to be approximately 7 times higher than baseline at 4 hours after clesrovimab-cfor dosing and maximum titers were reached by day 7, for a typical infant weighing 5 kg.
Exposure-Response Relationship
In the Phase 2b/3 trial evaluating the recommended dose of clesrovimab-cfor (a single dose of 105 mg) in healthy preterm and full-term infants (Trial 004), no significant relationship was observed between AUC (from day 1 to day 150) and clinical outcomes (e.g., RSV-associated Medically Attended Lower Respiratory Infection (MALRI)).Duration of Protection
Based on clinical trial data, the duration of protection demonstrated by a single dose of ENFLONSIA extends through 5 months [see Clinical Studies (14.2)].12.3 Pharmacokinetics
The PK of clesrovimab-cfor is approximately dose-proportional following a single IM administration of doses ranging from 20 mg to 210 mg in infants. Following IM administration of the 105 mg recommended dose, the geometric mean (% geometric CV) area under the time concentration curve from day 1 to day 150 (AUC0-150) is 6,470 mcg×d/mL (22.6%), peak concentration (Cmax) is 120 mcg/mL (25.4%), and the concentration at day 150 (C150) is 10.3 mcg/mL (36.6%). In the first RSV season, the clesrovimab-cfor serum exposures were similar in neonates and infants in Trial 004, in preterm neonates and infants born at less than or equal to 35 weeks GA (including less than 29 weeks GA) in Trial 007, and in neonates and infants with CLD or CHD in Trial 007.
Absorption
The median time to maximum concentration is 6.5 days (5.9, 7.4 which are the 2.5 and 97.5 percentiles, respectively).
Distribution
The estimated apparent volume of distribution for clesrovimab-cfor is 830 mL, for a typical infant weighing 5 kg.
Elimination
The clesrovimab-cfor terminal half-life is approximately 44.0 days and the estimated apparent clearance is 19.7 mL/day for a typical infant weighing 5 kg.
Metabolism
Clesrovimab-cfor is degraded into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics of clesrovimab-cfor were observed based on race or vulnerability to severe RSV disease (i.e., CLD, CHD, or GA <29 weeks). An effect of renal or hepatic impairment on clesrovimab-cfor pharmacokinetics is not expected.
Drug Interaction Studies
Since clesrovimab-cfor is eliminated by catabolism, no metabolic drug-drug interactions are expected. However, no formal drug interaction studies have been performed with ENFLONSIA.
Clinical Studies
Vaccines:
In clinical trials, when ENFLONSIA was given concomitantly with routine childhood vaccines, the safety profile of the co-administered regimen was generally comparable to the safety profile when ENFLONSIA and childhood vaccines were administered alone.12.4 Microbiology
Mechanism of Action
Clesrovimab-cfor is a recombinant human immunoglobulin G1 kappa (IgG1κ) neutralizing monoclonal antibody with a YTE triple amino acid substitution (M252Y/S254T/T256E) in the Fc region which increases binding to the neonatal Fc receptor leading to an extended serum half-life. Passive immunity is provided by clesrovimab-cfor, which targets the extracellular domain of the RSV fusion (F) protein to prevent fusion of the viral and cellular membranes and viral entry.
Clesrovimab-cfor binds to a conserved epitope on antigenic site IV on the F protein and binds to RSV A pre-fusion F glycoprotein and post-fusion F glycoprotein with equilibrium dissociation constant values (KD) of 71 pM and 480 pM, respectively.
Antiviral Activity
A neutralization assay in Hep-2 cells was used to determine clesrovimab-cfor potency against RSV A and RSV B isolates. Clesrovimab-cfor neutralized 47 RSV clinical isolates collected from North America locations between 1987 and 2016, with median EC50 values for RSV A isolates of 25 pM (3.71 ng/mL) (n=24; range of 1.2 to 74 pM [0.18 to 11.11 ng/mL]), and for RSV B isolates of 30 pM (4.48 ng/mL) (n=23; range of 4 to 198 pM [0.59 to 29.65 ng/mL]).
Clesrovimab-cfor also neutralized 12 contemporary isolates isolated from Texas, US, between 2016 and 2021, with a median EC50 value for RSV A isolates of 121 pM (18.02 ng/mL) (n=6; range of 59 to 186 pM [8.79 to 27.74 ng/mL]), and for RSV B isolates of 130 pM (19.41 ng/mL) (n=6; range of 95 to 153 pM [14.22 to 22.92 ng/mL]). The relatively high EC50 values for contemporary isolates compared with historical isolates is thought to be related to assay differences because the values for the control viruses also increased.
Antiviral Resistance
In Cell Culture
Clesrovimab-resistant RSV variants were identified after serial passage in cell culture of RSV A or RSV B in the presence of clesrovimab-cfor. Four variants were generated after 6 passages with RSV A, and one variant after 9 passages of RSV B. These variants each had reduced susceptibility to clesrovimab-cfor of >3,800-fold (RSV A) or >360-fold (RSV B) when assessed in cell culture, and harbored the following clesrovimab-cfor binding site substitutions: G446E, S443P+K445N, S443P+G446E, or S443P for the four RSV A variants, respectively, and S443P substitution for the RSV B variant.
In Surveillance Trials
In sequences reported in the GenBank® database (accessed April 15, 2024), the RSV binding epitope for clesrovimab-cfor was highly conserved (99.8%), with 13 polymorphisms identified at contact residues. Of these polymorphisms, the most common (I432T, 0.04% of sequences) conferred 4-fold (RSV A) or 1.6-fold (RSV B) reductions in susceptibility to clesrovimab-cfor in a cell culture neutralization assay. One polymorphism seen in 3 RSV A sequences, G446E, is a resistance-associated substitution selected in cell culture.
In a global surveillance study conducted between 2019 and 2023 in 8 countries across Northern and Southern Hemispheres, the clesrovimab-cfor binding site was highly conserved (>99%) in 300 RSV A and 255 RSV B sequences in clinical samples collected from individuals of various ages (<1 year to >60 years of age).
In Clinical Trials
In Trial 004, more substitutions in the clesrovimab-cfor binding site (amino acid positions 426 to 447) were observed at ≥3% variant allele frequency (VAF) in RSV infections of clesrovimab-cfor-treated participants (15/156 [9.6%]) compared with placebo participants (2/150 [1.3%]) from Day 1 through 180 post-dose. The majority of the binding site substitutions affected residue G446 (RSV A: G446E, G446R or G446W; and RSV B: G446E or G446R), and were seen at >50% VAF in at least one participant each. G446E, G446R, and G446W substitutions are resistance-associated, with G446E and G446W conferring >2,941-fold (RSV A) or >1,299-fold (RSV B) loss of susceptibility to clesrovimab-cfor, and G446R conferring >1,563-fold (RSV A) loss of susceptibility to clesrovimab-cfor (RSV B not assessed). Other binding site substitutions seen in clesrovimab-cfor-treated participants at <10% VAF were RSV B: F435S, S443L, G446V, and V447I (no cell culture neutralization data available).
In Trial 007, RSV infections with binding site substitutions at >50% VAF included RSV A (G446W) and RSV B (G446E and G446R), in clesrovimab-cfor-treated participants.
For Trials 004 and 007, there was no clear association of binding site substitutions seen in RSV infections and RSV-associated Medically Attended Lower Respiratory Infection (MALRI) or hospitalization. However, for substitutions seen at high VAF% from Days 1 to 150 post-dose, one participant in Trial 004 with RSV A G446W substitution had RSV-associated hospitalization, and one participant in Trial 007 with RSV B G446R substitution had RSV-associated severe MALRI and hospitalization.
Cross-resistance
No cross-resistance was seen for RSV variants harboring palivizumab or nirsevimab resistance-associated substitutions in cell culture neutralization assays. Clesrovimab-cfor did not lose activity against RSV A or RSV B clinical isolates with palivizumab resistance-associated substitution N262Y, or recombinant RSV B with nirsevimab resistance-associated substitutions N208S, I64T+K68E, or I64T+K68E+I206M+Q209R, which were observed in clinical trials of nirsevimab. Not all nirsevimab resistance-associated substitutions have been assessed for cross-resistance with clesrovimab-cfor. Both nirsevimab and palivizumab neutralized RSV A and B variants harboring clesrovimab-cfor resistance-associated substitutions G446E or G446W in cell culture.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies.
In Trial 004 and Trial 007, after receiving the approved recommended dose in RSV season 1, 6% (120/2112) and 5% (13/291) of participants were ADA-positive on Day 150, respectively; and 12% (124/1033) and 13% (34/261) of participants were ADA-positive through Day 240, respectively.
The impact of ADA on efficacy is unknown due to the low rates of MALRI and ADA. There was no identified impact of ADA on pharmacokinetics, RSV serum neutralizing activity or safety of ENFLONSIA during RSV season 1.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Description of Clinical Trials
The efficacy and safety of ENFLONSIA were evaluated in preterm and full-term infants in the trials summarized in Table 2.
Table 2: Trials Conducted with ENFLONSIA for the Prevention of Medically Attended RSV Lower Respiratory Tract Disease Trial Study Population Study Arms* GA=gestational age; CLD=chronic lung disease; CHD=hemodynamically significant congenital heart disease - * Participants randomized and treated
- † 1 participant was randomized to placebo but received ENFLONSIA
Trial 004
(NCT04767373)Infants born at ≥29 weeks GA from birth up to 1 year entering their first RSV season. ENFLONSIA (N=2,411)
Placebo (N=1,203)†Trial 007
(NCT04938830)Infants born at ≤35 weeks GA, or infants with CLD of prematurity or hemodynamically significant CHD from birth up to 1 year entering their first RSV season. ENFLONSIA (N=446)
Palivizumab (N=450)14.2 Prevention of RSV-Associated Disease in Neonates and Infants (≥29 Weeks GA) Entering Their First RSV Season (Trial 004)
Trial 004 was a Phase 2b/3, randomized, double-blind placebo-controlled, multi-site trial conducted in 22 countries from the Northern and Southern Hemispheres to evaluate the efficacy of ENFLONSIA in early and moderate preterm infants (≥29 to <35 weeks GA) and late preterm and full-term infants (≥35 weeks GA). The trial assessed the efficacy of ENFLONSIA in the prevention of RSV-associated disease across a spectrum of severity. Participants were randomized 2:1 to receive a single 105 mg dose of ENFLONSIA or saline placebo by IM injection.
Among participants who received ENFLONSIA or saline placebo, the median age of infants was 3.1 months (range: 0 to 12 months); 80% were less than 6 months; 16% were greater than or equal to 6 to less than 9 months, 4% were greater than or equal to 9 months of age, and 51% were male. Of these participants, 18% were GA greater than or equal to 29 weeks and less than 35 weeks, and 82% were GA greater than or equal to 35 weeks. The racial distribution was as follows: 45% were White; 27% were Asian; 14% were Black or African American; 12% were Multi-racial and 2% were American Indian or Alaska Native; 28% were of Hispanic or Latino ethnicity.
The primary endpoint was the incidence of RSV-associated Medically Attended Lower Respiratory Infection (MALRI) characterized as cough or difficulty breathing and requiring ≥1 indicator of LRI (wheezing, rales/crackles) or severity (chest wall in-drawing/retractions, hypoxemia, tachypnea, dehydration due to respiratory symptoms) through 150 days after dosing. Medically Attended (MA) includes all healthcare provider visits in settings such as outpatient clinic, clinical study site, emergency department, urgent care center, and/or hospital. The statistical criterion for success required the lower bound of the 95% CI of efficacy to be greater than 25%. RSV-associated hospitalization through 150 days after dosing was evaluated as a key secondary endpoint. The statistical criterion for success required the lower bound of the 95% CI of efficacy to be greater than 0%. Both efficacy endpoints required an RSV-positive RT-PCR nasopharyngeal (NP) sample.
Table 3 displays efficacy results for the primary and key secondary RSV-associated disease endpoints in preterm and full-term infants from days 1 through 150 post-dose.
Table 3: Incidence of RSV-Associated Disease in Infants Born at ≥29 Weeks GA Days 1 Through 150 Post-Dose (Trial 004) RSV-Associated Endpoint ENFLONSIA
(n=2,411)Placebo
(n=1,203)Efficacy* (95% CI)† Number
of casesIncidence
Rate
over 5 monthsNumber
of casesIncidence
Rate
over 5 monthsn=Number of participants eligible for inclusion in the full analysis set population. - * Efficacy for MALRI (requiring ≥1 indicator of LRI or severity) and hospitalization based on relative risk reduction against placebo adjusted for hemisphere at randomization, gestational age group and age group at randomization.
- † Estimate and 95% CI of efficacy were estimated from the modified Poisson regression with robust variance method.
- ‡ (p<0.001)
MALRI (requiring ≥1 indicator of LRI or severity) 60 0.026 74 0.065 60.5% (44.2, 72.0)‡ Hospitalization 9 0.004 28 0.024 84.3% (66.7, 92.6)‡ 14.3 Prevention of RSV-Associated Disease in Infants Born at ≤35 Weeks Gestational Age and Infants with CLD of Prematurity or Hemodynamically Significant CHD Entering Their First RSV Season (Trial 007)
Trial 007 is a Phase 3, randomized, partially-blind, palivizumab-controlled, multi-site trial conducted in 27 countries from the Northern and Southern Hemispheres to evaluate the efficacy of ENFLONSIA in early (<29 weeks GA) or moderate preterm infants (≥29 to ≤35 weeks GA), and infants with chronic lung disease of prematurity or congenital heart disease of any GA, who are at increased risk for severe RSV disease. Participants were randomized to receive ENFLONSIA or palivizumab by IM injection. Participants randomized to ENFLONSIA received a single 105 mg dose on Day 1 followed by a dose of placebo one month later; 15 mg/kg palivizumab was administered on Day 1 and every month thereafter for a total of 3 to 5 doses.
Among participants who received ENFLONSIA or palivizumab, the median age of infants was 2.5 months (range: 0 to 12 months); 89% were less than 6 months; 9% were greater than or equal to 6 to less than 9 months, 2% were greater than or equal to 9 months of age; and 50% were male. Of these participants, 28% had CLD, 11% had CHD, 6% were GA less than 29 weeks with neither CLD nor CHD and 55% were GA greater than or equal to 29 weeks with neither CLD nor CHD. The racial distribution was as follows: 52% were White; 18% were Asian; 15% were Black or African American; 12% were Multi-racial, and 1% were American Indian or Alaska Native; 32% were of Hispanic or Latino ethnicity.
The efficacy of ENFLONSIA in infants at increased risk for severe RSV disease, including preterm infants and infants with chronic lung disease of prematurity or congenital heart disease was established by extrapolation of efficacy of ENFLONSIA from Trial 004 to Trial 007 based on similar pharmacokinetic exposure [see Clinical Pharmacology (12.3)]. The incidence rate of RSV-associated MALRI (requiring ≥1 indicator of LRI or severity) through 150 days after dosing was generally comparable between ENFLONSIA (incidence rate=3.6%, 95% CI: 2.0, 6.0) and palivizumab (incidence rate=2.9%, 95% CI: 1.5, 5.2). The incidence rate of RSV-associated hospitalization through 150 days after dosing was generally comparable between ENFLONSIA (incidence rate=1.3%, 95% CI: 0.4, 2.9) and palivizumab (incidence rate=1.5%, 95% CI: 0.5, 3.2).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ENFLONSIA injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution supplied as follows:
Carton containing one or ten single-dose prefilled type I glass syringe(s) with Luer Lock and plunger stopper. The prefilled syringe is not made with natural rubber latex.
Prefilled syringe Pack Size NDC 105 mg/0.7 mL single-dose Carton of 1 0006-5073-01 105 mg/0.7 mL single-dose Carton of 10 0006-5073-02 Storage and Handling
- Store prefilled syringes under refrigeration at 36°F to 46°F (2°C to 8°C).
- Keep the prefilled syringe in the original carton to protect from light until time of use.
- ENFLONSIA may be kept at room temperature between 68°F to 77°F (20°C to 25°C) for a maximum of 48 hours. After removal from the refrigerator, ENFLONSIA must be used within 48 hours or discarded.
- Do not freeze. Do not shake.
-
17 PATIENT COUNSELING INFORMATION
Advise the child’s caregiver to read the FDA-approved patient labeling (Patient Information).
Hypersensitivity Reactions Including Anaphylaxis
Inform the patient’s caregiver of the signs and symptoms of potential hypersensitivity reactions, and advise the caregiver to seek medical attention immediately if the infant experiences a hypersensitivity reaction to ENFLONSIA [see Warnings and Precautions (5.1)].
Dosage and Administration
Advise the caregiver that the infant will receive ENFLONSIA by IM injection by a healthcare provider [see Dosage and Administration (2.1)].
-
SPL UNCLASSIFIED SECTION
Manufactured by:
Merck Sharp & Dohme LLC
Rahway, NJ 07065, USAU.S. license number 0002
For patent information: www.msd.com/research/patent
Copyright © 2025 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.uspi-mk1654-i-2506r001
-
PATIENT PACKAGE INSERT
Patient Information
ENFLONSIA™ (en-flahn-see-ah)
(clesrovimab-cfor)
injection, for intramuscular useThis Patient Information has been approved by the U.S. Food and Drug Administration Issued: 06/2025 What is ENFLONSIA?
ENFLONSIA is a prescription medicine to help prevent lung disease, including severe lung disease, caused by Respiratory Syncytial Virus (RSV) in newborns and babies who are born during or entering their first RSV season.
ENFLONSIA contains antibodies to help prevent RSV disease.
It is not known if ENFLONSIA is safe and effective in children older than 12 months of age.
Your child should not receive ENFLONSIA if your child has a history of serious allergic reactions to any of the ingredients in ENFLONSIA. See the end of this Patient Information leaflet for a complete list of ingredients in ENFLONSIA. Before your child receives ENFLONSIA, tell their healthcare provider about all of your child’s medical conditions, including if your child: - is scheduled to have heart surgery during or entering their first RSV season.
How is ENFLONSIA given? - ENFLONSIA is given as an injection, in the thigh muscle, by your child’s healthcare provider.
- Your child should get ENFLONSIA before the start of or during the RSV season. RSV season is the time of year when RSV infections are most common, usually occurring fall through spring. Your child’s healthcare provider can tell you when the RSV season starts in your area.
- Your child may still get RSV disease after receiving ENFLONSIA. Talk to your child’s healthcare provider about what symptoms to look for.
- If your child has heart surgery, your child’s healthcare provider may need to give your child an additional ENFLONSIA injection soon after surgery.
- Your child can get ENFLONSIA at the same time as routine childhood vaccines.
What are the possible side effects of ENFLONSIA? - Serious allergic reactions have happened with other medicines like ENFLONSIA. Get medical help right away if your child has any of the following signs or symptoms of a serious allergic reaction, including:
- swelling of the face, mouth, or tongue
- difficulty swallowing or breathing
- unresponsiveness
- bluish color of skin, lips or under fingernails
- muscle weakness
- severe rash, hives or itching
The most common side effects of ENFLONSIA are:
- redness and swelling at the site of your child’s injection
- rash
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of ENFLONSIA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or child’s healthcare provider for information about ENFLONSIA that is written for health professionals.What are the ingredients in ENFLONSIA?
Active ingredient: clesrovimab-cfor
Inactive ingredients: arginine hydrochloride, histidine, L-histidine monohydrochloride monohydrate, polysorbate 80, sucrose and water for injection.Manufactured by: Merck Sharp & Dohme LLC, Rahway, NJ 07065, USA. U.S. License No. 0002 For patent information: www.msd.com/research/patent. Copyright © 2025 Merck & Co., Inc., Rahway, NJ, USA, and its affiliates.
All rights reserved.usppi-mk1654-i-2506r000 For more information, go to www.ENFLONSIA.com or call 1-877-888-4231. -
PRINCIPAL DISPLAY PANEL - 1 Single-Dose Syringe Carton
NDC: 0006-5073-01
One 105 mg/0.7 mL single-dose
prefilled syringeENFLONSIA™
(clesrovimab-cfor) Injection105 mg / 0.7 mL
For Intramuscular Use Only
This package does not contain a needle.
Must be administered by a healthcare provider.REFRIGERATE
Rx Only
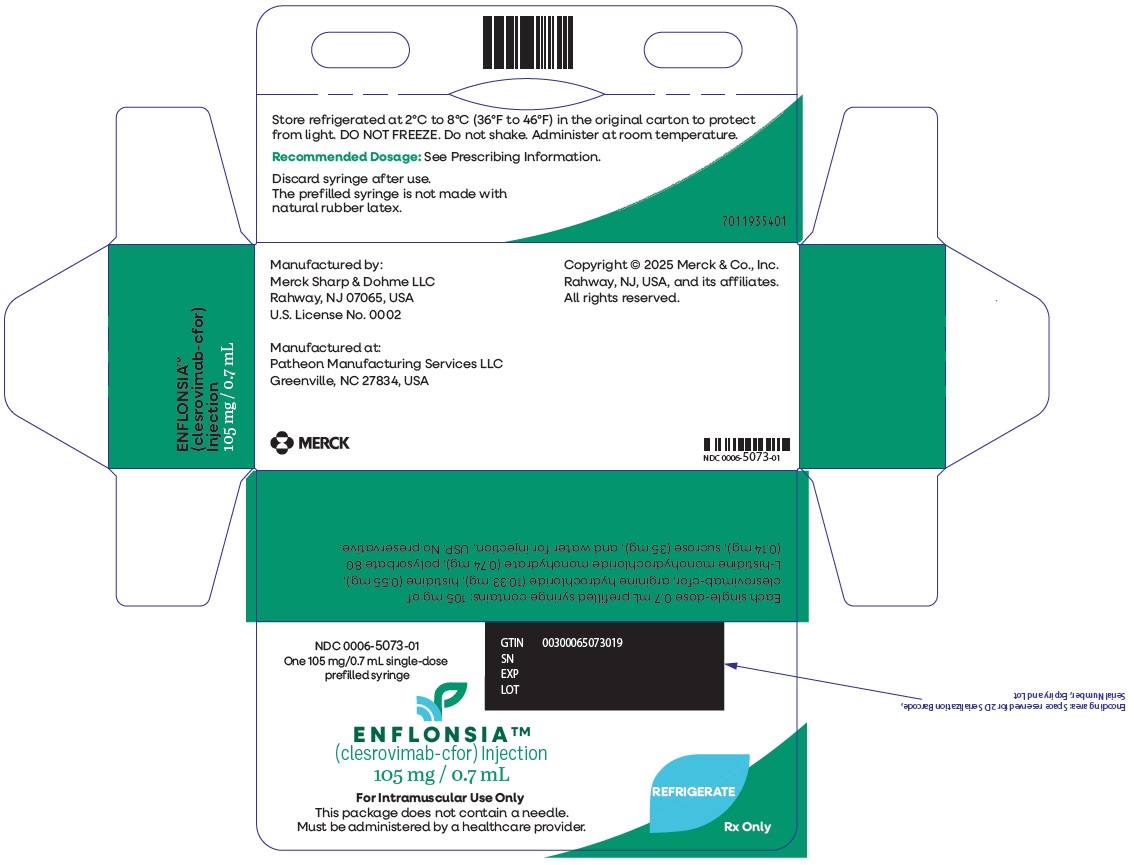
-
PRINCIPAL DISPLAY PANEL - 10 Single-Dose Syringe Carton
NDC: 0006-5073-02
Ten 105 mg/0.7 mL single-dose
prefilled syringesENFLONSIA™
(clesrovimab-cfor)
Injection105 mg / 0.7 mL
For Intramuscular Use Only
This package does not contain needles.
Must be administered by a healthcare provider.REFRIGERATE
Rx only
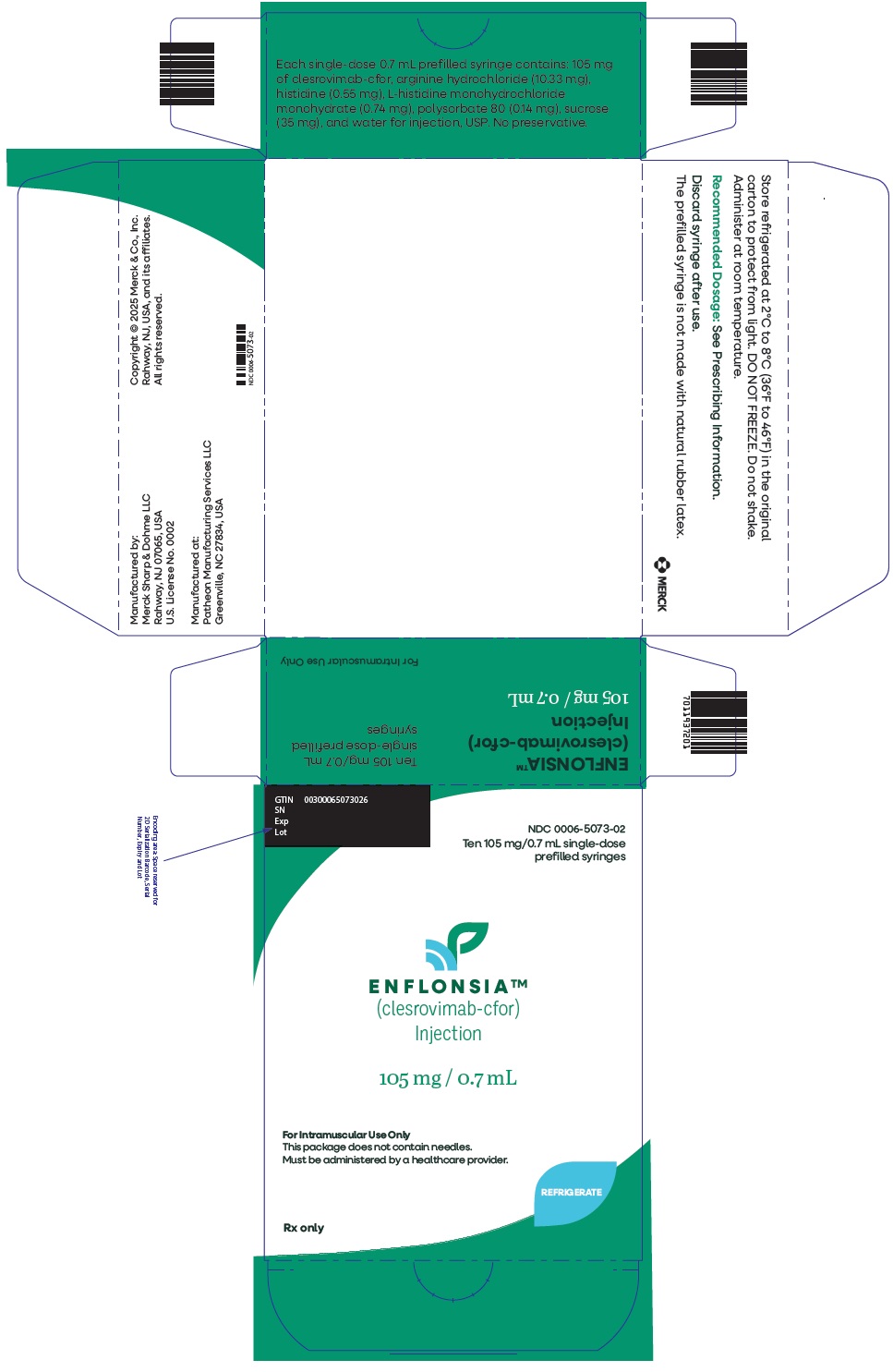
-
PRINCIPAL DISPLAY PANEL - 0.7 mL Syringe Label
NDC: 0006-5073-99
One 105 mg/0.7 mL single-dose
prefilled syringeENFLONSIA™
(clesrovimab-cfor)
Injection105 mg / 0.7 mL
For Intramuscular Use Only
Rx onlyStore at 2°C-8°C (36°F-46°F).
Manuf. by: Merck Sharp & Dohme LLC
U.S. License No. 0002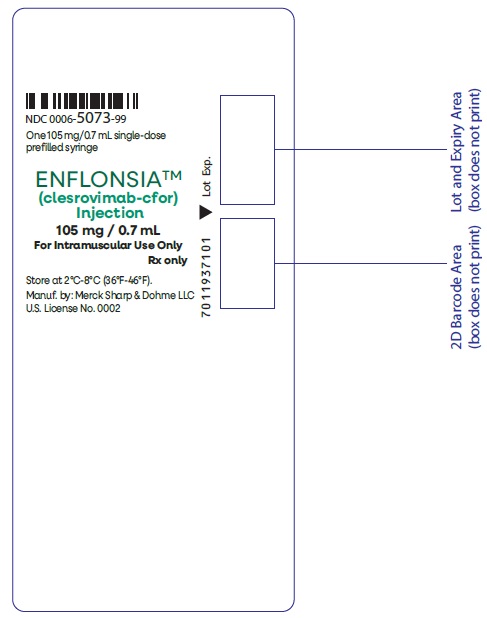
-
INGREDIENTS AND APPEARANCE
ENFLONSIA
clesrovimab injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0006-5073 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLESROVIMAB (UNII: VJO29R4CP9) (CLESROVIMAB - UNII:VJO29R4CP9) CLESROVIMAB 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength ARGININE HYDROCHLORIDE (UNII: F7LTH1E20Y) HISTIDINE HYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) HISTIDINE (UNII: 4QD397987E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (colorless to slightly yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0006-5073-01 1 in 1 CARTON 06/09/2025 1 NDC: 0006-5073-99 0.7 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 0006-5073-02 10 in 1 CARTON 06/09/2025 2 NDC: 0006-5073-99 0.7 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761432 06/09/2025 Labeler - Merck Sharp & Dohme LLC (118446553)
Trademark Results [ENFLONSIA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ENFLONSIA 97722844 not registered Live/Pending |
Merck Sharp & Dohme LLC 2022-12-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.