STERITALC 4G- talc powder STERITALC 2G- talc powder STERITALC 3G- talc powder
Steritalc by
Drug Labeling and Warnings
Steritalc by is a Prescription medication manufactured, distributed, or labeled by Novatech SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use STERITALC® safely and effectively. See full prescribing information for STERITALC®.
STERITALC® (talc), powder, for intrapleural use
Initial U.S. Approval: 2003RECENT MAJOR CHANGES
Warnings and Precautions (5.4) 06/2025
INDICATIONS AND USAGE
___________ INDICATIONS AND USAGE____________
STERITALC® is a sclerosing agent indicated: (1)- To decrease the recurrence of malignant pleural effusions in symptomatic patients following maximal drainage of the pleural effusion. (1.1)
- In adults to decrease the recurrence of pneumothorax. (1.2)
DOSAGE AND ADMINISTRATION
________ DOSAGE AND ADMINISTRATION________ (2)
- Malignant Pleural Effusion: The recommended dose is 2 to 5 grams administered intrapleurally. (2.2)
- Pneumothorax: The recommended dose is 2 grams administered intrapleurally.
- Do not exceed a total cumulative dosage of 10 grams per procedure. (2.3)
- Prepare and administer as recommended. (2.5)
DOSAGE FORMS AND STRENGTHS
_______ DOSAGE FORMS AND STRENGTHS_______ (3)
- Powder: 2 grams, in a 50 mL single-dose vial (3)
- Powder: 4 grams, in a 50 mL single-dose vial (3)
- Powder: 3 grams, in a 10 mL single-dose vial (3)
WARNINGS AND PRECAUTIONS
_ ______WARNINGS AND PRECAUTIONS_____________ (5)
- Pneumonitis and Acute Respiratory Distress Syndrome (ARDS): Acute Pneumonitis and ARDS, have been reported with intrapleural use of various talc products. (5.1)
- Interference with Future Procedures: Sclerosis of the pleural space may preclude or complicate subsequent ipsilateral surgery and diagnostic procedures. (5.2)
- Lead Content: STERITALC® contains lead as an impurity. May cause lead toxicity, especially in children. (5.3)
- Asbestos Content: Talc products may contain trace amounts of asbestos. Exposure to asbestos may increase the risk of mesotheliomas and other cancers. (5.4, 11)
(5)
ADVERSE REACTIONS
USE IN SPECIFIC POPULATIONS
------------USE IN SPECIFIC POPULATIONS-------------- (7)
- Females and Males of Reproductive Potential: Advise females of reproductive potential to use effective contraception. (8.3)
- Lactation: Advise women not to breastfeed. (8.2)
Revised: 12/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1.INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
6. ADVERSE REACTIONS
8. USE IN SPECIFIC POPULATIONS
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
13. NONCLINICAL TOXICOLOGY
14. CLINICAL STUDIES
16. HOW SUPPLIED/STORAGE AND HANDLING
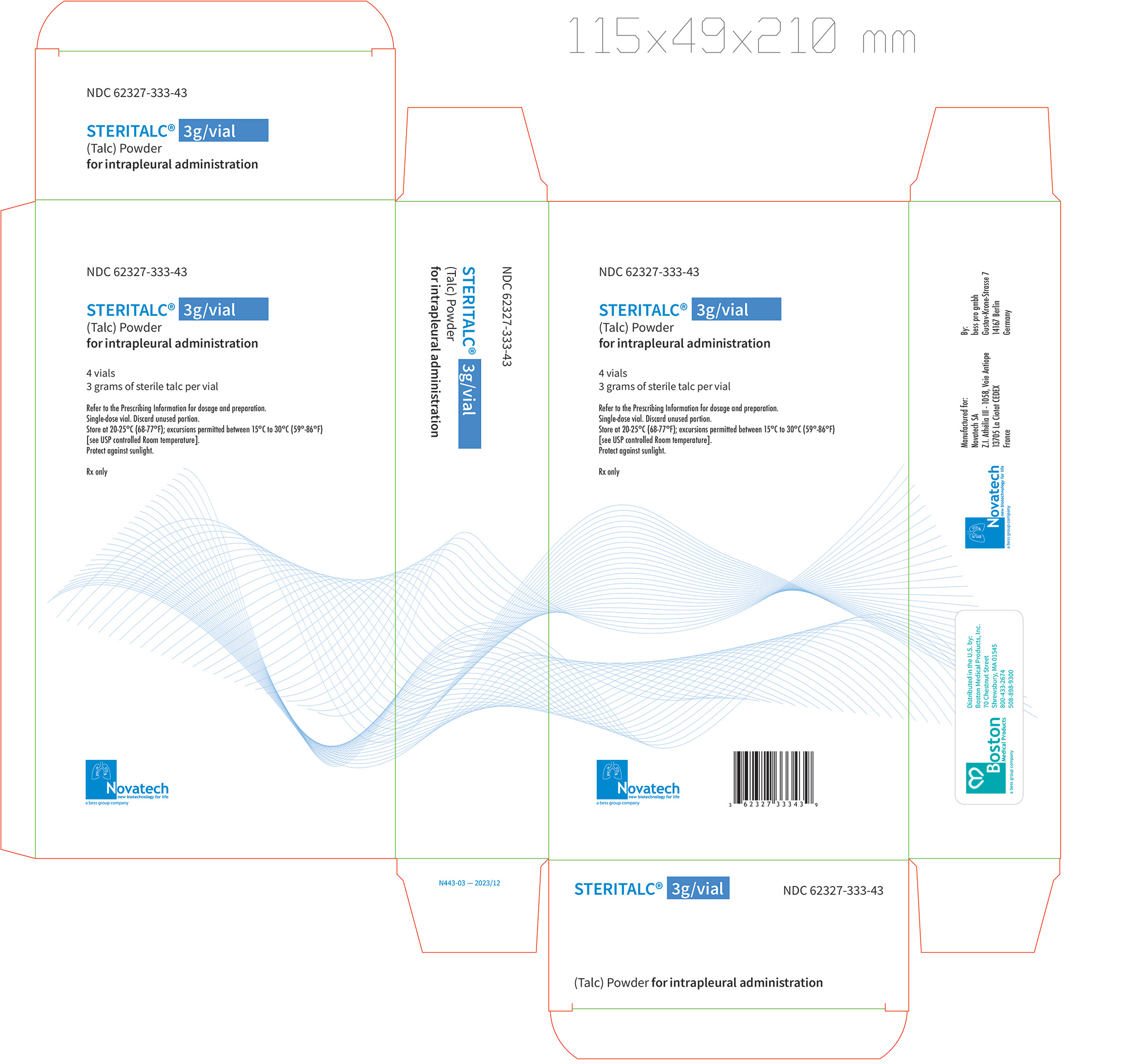
Principal Display Label, Steritalc 3g vial label
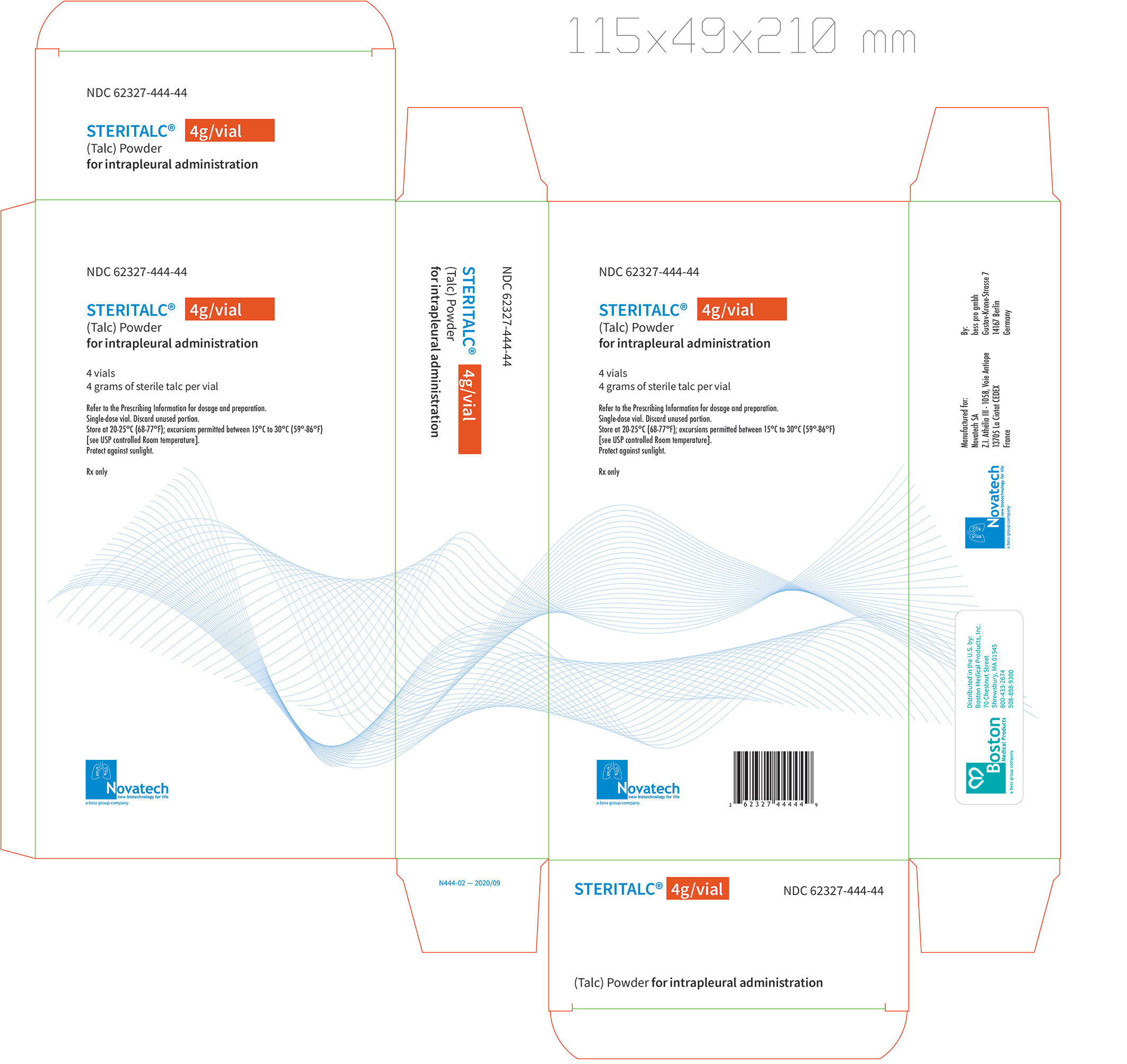
Principal Display Label, Steritalc 4g vial label
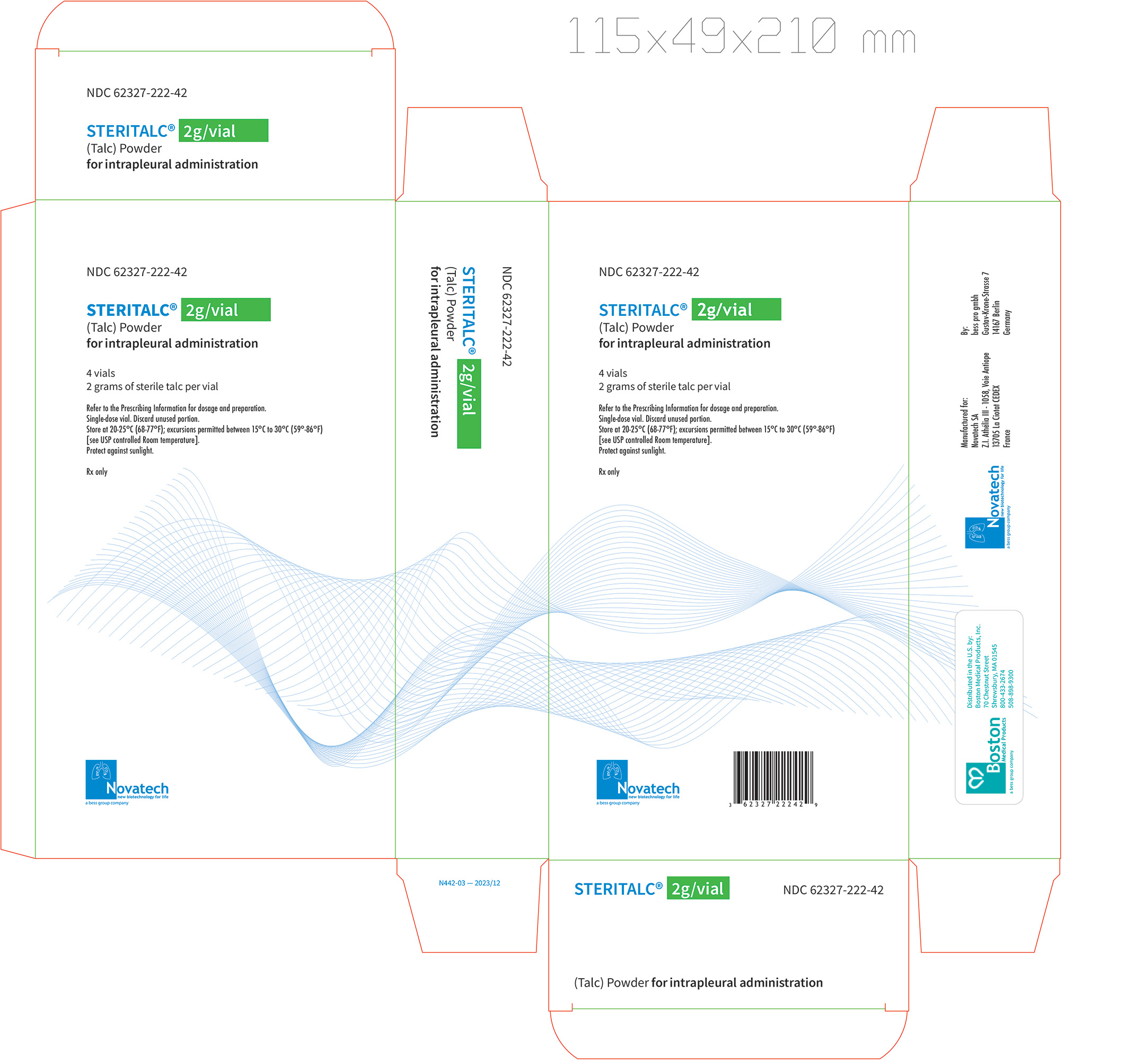
Principal Display Label, Steritalc 2g vial label
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1.INDICATIONS AND USAGE
1.INDICATIONS AND USAGE
1.1 Malignant Pleural Effusion
STERITALC is indicated to decrease the recurrence of malignant pleural effusions in symptomatic patients following maximal drainage of the pleural effusion.
1.2 Pneumothorax
STERITALC is indicated in adults to decrease the recurrence of pneumothorax.
-
2. DOSAGE AND ADMINISTRATION
2.1 Important Use Information
STERITALC is for pleurodesis only - Do NOT administer STERITALC intravenously.
Administer STERITALC after adequate drainage of the pleural effusion or air.
2.2 Recommended Dose for Malignant Pleural Effusion
The recommended dose for malignant pleural effusion is 2 to 5 grams administered intrapleurally. According to the physician’s discretion, and in consideration of diagnosis and patient’s condition, different dosages may be applied, but a total dosage of 10 grams should not be exceeded.
2.3 Recommended Dose for Pneumothorax
The recommended dose for pneumothorax is 2 grams administered intrapleurally. According to physician’s discretion and in consideration of diagnosis and patient’s condition, different dosages may be applied, but a cumulative dosage of 10 grams should not be exceeded.
2.4 Preparation
Slurry for Tube Thoracostomy
STERITALC 2 grams and 4 grams dosage forms
Do not prepare the slurry in advance. Use the slurry immediately after preparation.
Prepare the talc suspension using aseptic technique in an appropriate laminar flow hood as follows:
Step 1: Fully bend or remove the flap into the direction of the arrow. The top can now be punctured to mix the slurry.
Step 2: Using a 16 gauge needle attached to a 60-mL Luer Lok syringe, draw up 50 mL of 0.9 % Sodium Chloride injection, USP. Vent the talc bottle using a needle. Slowly inject the 50 mL of 0.9% Sodium Chloride Injection, USP into the glass vial.
Step 3: Swirl the glass vial to disperse the talc powder.
Step 4: Divide the contents of the glass vial equally into two 60-mL Luer Lok syringes, each attached with a 16 gauge needle, by withdrawing 25 mL of the suspension into each syringe with continuous swirling. Add 0.9% Sodium Chloride Injection, USP to a total volume of 50 mL in each syringe. Draw 10 mL of air into each syringe to the 60 mL mark to serve as a headspace for mixing prior to administration.
For STERITALC 2 grams, each syringe should contain 1 gram of Sterile Talc Powder in 50 mL of 0.9% Sodium Chloride Injection, USP with an air headspace of 10 mL.
For STERITALC 4 grams, each syringe should contain 2 grams of Sterile Talc Powder in 50 mL of 0.9% Sodium Chloride Injection, USP with an air headspace of 10 mL.
Step 5: Label the syringes with the talc concentration, the expiration date and time, the identity of the patient intended to receive the material, and the following statements:
“SHAKE SYRINGE WELL to resuspend before administration”
“FOR PLEURODESIS ONLY – not for intravenous administration”
Step 6: If not used immediately, store prepared suspension in refrigerator. Discard the prepared suspension if not used within 12 hours.
Insufflation/Poudrage
STERITALC 2 grams and 4 grams dosage forms
Use an FDA-approved or cleared device for STERITALC insufflation/poudrage.
Step 1: Fully remove the top from the vial.
Step 2: Fill the content into the applicator for insufflation/poudrage.
Step 3: Follow the selected device manufacturer's instructions for insufflation/poudrage.
STERITALC 3 grams dosage form
STERITALC 3 grams is designed to be used for the administration of talc in combination with a compatible FDA approved or FDA cleared device intended for manual insufflation of medical grade talc into the pleural cavity during pleurodesis such as Novatech SA’s NOVATECH® TALCAIR™.
Step 1: Remove the aluminum tear-off cap from the vial.
Step 2: Remove the stopper from the vial.
Step 3: Close the vial with the vial coupling.
Step 4: Firmly press the vial coupling onto the vial until you feel and hear the lid "click" onto the vial top. To do this, place the vial on a firm and level base.
Step 5: Connect the insufflation bulb to the vial coupling using the Luer lock connector.
Step 6: Ensure that all components are firmly connected with each other.
2.5 Administration
Slurry for Tube Thoracostomy
STERITALC 2 grams and 4 grams dosage forms
Prior to administration, continuously agitate the syringes to evenly redisperse the talc and avoid settlement. Immediately prior to administration, vent the 10 mL air headspace from each syringe. Administer the talc suspension through the chest tube according to standard procedures.
Step 1: Inject the slurry through the pleural drainage into the pleural cavity.
Step 2: Clamp the pleural drainage. Keep the negative pressure in the pleural cavity.
Step 3: While the slurry remains in the pleural cavity, reposition the patient regularly to achieve even distribution of the slurry.
Step 4: Aspirate the slurry through the pleural drainage.
Insufflation/Poudrage
Perform intervention by means of a tube thoracoscopy. Follow the selected device manufacturer's instructions for insufflation/poudrage.
STERITALC 2 grams and 4 grams dosage forms
Step 1: Introduce the cannula into the trocar.
Step 2: Distribute the talc evenly in the pleural cavity. To do this, carefully spray several times. After spraying a few times, change the cannula direction.
After use, some talc may remain in the vial. Discard unused portion.
STERITALC 3 grams dosage form
Keep the product upright during use. Avoid contact between the cannula tip and tissue/body fluids. Otherwise the cannula may be blocked. In case of blockage: Shorten the cannula with a scalpel to be able to continue the intervention. Cut perpendicularly to the cannula and ensure that the cutting edge is straight and free of burs.
Step 1: Introduce the cannula into the trocar.
Step 2: Distribute the talc evenly in the pleural cavity. To do this, press the insufflation bulb carefully and at regular intervals. After spraying a few times, change the cannula direction.
After use, some talc may remain in the vial. Discard unused portion
-
3. DOSAGE FORMS AND STRENGTHS
White or off-white to light gray sterile powder provided in the following strengths:
- Powder, for intrapleural use: STERITALC, 2 grams in a 50 mL single-dose vial
- Powder, for intrapleural use: STERITALC, 4 grams in a 50 mL single-dose vial
- Powder, for intrapleural use: STERITALC, 3 grams in a 10 mL single-dose vial, for use with Novatech SA’s NOVATECH® TALCAIR™
- 4. CONTRAINDICATIONS
-
5. WARNINGS AND PRECAUTIONS
5.1 Pneumonitis and Acute Respiratory Distress Syndrome (ARDS)
Acute Pneumonitis and ARDS, including fatal cases, have been reported with intrapleural use of various talc products. Systemic exposure of talc could be affected by the integrity of the visceral pleura and could be increased if talc is administered immediately after mechanical abrasion or biopsy of the pleura. The literature also suggests a possible correlation between talc particle size distribution and risk of toxicity.
There are published reports of two large, prospective trials conducted to evaluate the safety of STERITALC administered intrapleurally. One trial evaluated 558 patients treated with STERITALC 4g by poudrage for MPE. The second trial evaluated 418 patients with recurrent primary spontaneous pneumothorax treated with STERITALC 2 g by poudrage. No cases of ARDS or talc-related lung injury were reported.
5.2 Interference with Future Procedures
Sclerosis of the pleural space may preclude or complicate subsequent ipsilateral surgery and diagnostic procedures. Consider the possible effects of the use of STERITALC on future diagnostic and therapeutic procedures prior to administration.
5.3 Lead Content
Lead is present in STERITALC as an impurity. The main target organ for lead toxicity is the nervous system, but effects of lead exposure also include increased blood pressure, anemia, decreased sperm production, and damage to the kidneys in children and adults. Minimal risk levels of lead have not been derived for humans because clear thresholds for effects have not been identified.
Children are more sensitive to lead toxicity than adults, and no safe blood level has been determined in children. Cognitive and neurobehavioral deficits are observed in children exposed to lead. Exposure of a pregnant woman to lead may cause miscarriage, premature birth, lower birth weights and slow or impaired mental development in the child.
Administration of STERITALC at the highest recommended dose of 10 grams may deliver up to 40 mcg of lead.
5.4 Asbestos Content
Talc products may contain trace amounts of asbestos. Asbestos is a known human carcinogen. Exposure to asbestos may increase the risk of mesotheliomas and other cancers including lung, larynx, and ovarian cancer.
STERITALC is tested for asbestos and asbestiform fibers [see DESCRIPTION (11)].
-
6. ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling.
- Pneumonitis and Acute Respiratory Distress Syndrome (ARDS) [see Warnings and Precautions (5.1)]
- Lead Content [see Warnings and Precautions (5.3)]
- Asbestos Content [see Warnings and Precautions (5.4)]
Common adverse reactions observed with intrapleural use of various talc products are fever and pain.
Other adverse reactions include dyspnea, arrhythmia, empyema, pneumonitis and acute respiratory distress syndrome (ARDS).
Procedure related adverse reactions such as bleeding, hemothorax, wound infections, atelectasis and pneumonia may occur.
-
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
STERITALC is contraindicated for use in pregnant women because it contains lead, which can cause fetal harm and potential loss of pregnancy [see Warnings and Precautions (5.3)]. There are no human data on the use of STERITALC in pregnant women. In an animal reproduction study, oral administration of talc in pregnant rabbits during organogenesis revealed no evidence of teratogenicity at dose up to approximately 5 times the human dose [see Data].
Data
Animal Data
In an embryo-fetal developmental toxicity study in rabbits, talc was administered to rabbits by oral gavage daily during the period of organogenesis at doses up to 900 mg/kg (approximately 5 times the human dose on a mg/m2 basis). No significant dose-related toxicity was reported except at maternally toxic doses. In multiple animal studies, intrapleurally administered talc was not absorbed systemically.
8.2 Lactation
Risk Summary
There is no information regarding the presence of talc in human milk, the effects on the breastfed infants, or the effects on milk production. STERITALC contains lead, which is known to have adverse effects on health and development in neonates, infants and children [see Warnings and Precautions (5.3)]. Because of the potential for serious adverse reactions in breast-fed infants from lead content in STERITALC, advise lactating women not to breastfeed during treatment with STERITALC and for 5 months after the final dose.
8.3 Females and Males of Reproductive PotentialContraception
STERITALC contains lead, which can cause fetal harm when administered to a pregnant woman [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment and for 5 months following the final dose of STERITALC.
Infertility
STERITALC contains lead, which may impair fertility in males of reproductive potential [see Warnings and Precautions (5.3)].
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients. Lead is present in STERITALC as an impurity [see Warnings and Precautions (5.3)].
-
11. DESCRIPTION
STERITALC (talc) is a sclerosing agent for intrapleural administration. The molecular formula for talc is Mg3Si4O10(OH)2. The molecular weight is 379.3 g/mole. The chemical name of talc is hydrated magnesium silicate.
The talc powder contains ≥ 95% of hydrated magnesium silicate; associated minerals include chlorites (hydrated aluminum and magnesium silicates), magnesite (magnesium carbonate), calcite (calcium carbonate), and dolomite (calcium and magnesium carbonate). The talc elementary sheet is composed of a layer of magnesium-oxygen/hydroxyl octahedra, sandwiched between two layers of silicon-oxygen tetrahedra. Talc is insoluble in water.
STERITALC is tested for asbestos and asbestiform fibers consistent with USP standards for talc-containing products.
STERITALC is supplied as 2 grams, 3 grams, and 4 grams white or off-white to light gray, sterile powder. STERITALC contains particle size-controlled talc, graded to decrease the proportion of smaller particles.
- 12. CLINICAL PHARMACOLOGY
-
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies on the carcinogenicity of talc have been performed using non-standard designs which prevent firm conclusions on its carcinogenicity. With single intraperitoneal administration to mice at 20 mg and observation for at least 6 months, or 4 weekly doses administered intraperitoneally at 25 mg/dose to rats with observation for at least 84 weeks, tumor incidence was not increased. In these studies the talc and its asbestos content were not characterized.
Genotoxicity was tested in cultures of rat pleural mesothelial cells (RPMC) for unscheduled DNA synthesis (UDS) and sister chromatid exchanges (SCEs). None of the talc samples induced enhancement of UDS or SCEs in treated cultures.
No information is available on impairment of fertility in animals by talc.
-
14. CLINICAL STUDIES
14.1 Malignant Pleural Effusion
The data demonstrating safety and efficacy of talc in the treatment of malignant pleural effusions are derived from the published medical literature. In these studies, greater than 1000 patients with malignant pleural effusions have been reported (with varying degrees of detail and durations of response) to have had successful pleurodesis with talc, administered either by poudrage or as a slurry via chest tube. There are published efficacy data for more than 200 patients treated with STERITALC for malignant pleural effusion, with a success rate of approximately 89% (range 73-91%).
14.2 Pneumothorax
The data demonstrating safety and efficacy of talc in the treatment of pneumothorax are derived from the published medical literature. There are published efficacy data for more than 500 patients treated with STERITALC for pneumothorax, some in conjunction with surgical procedures, with successful pleurodesis rates of 97-100%.
-
16. HOW SUPPLIED/STORAGE AND HANDLING
STERITALC, white or off-white to light gray, sterile powder is supplied as
- two grams of talc powder in a single-dose 50 mL Type III glass vial, closed with a stopper, and crimped with an aluminum cap; with 4 vials packaged in one carton.
- NDC: 62327-222-02 (vial)
NDC: 62327-222-42 (carton)
four grams of talc powder in a single-dose 50 mL Type III glass vial, closed with a stopper, and crimped with an aluminum cap; with 4 vials packaged in one carton.
NDC: 62327-444-04 (vial)
NDC: 62327-444-44 (carton)- three grams of talc powder in a single-dose 10 mL Type I brown glass vial, closed with a stopper, and crimped with an aluminum cap; with 4 vials packaged in one carton.
NDC: 62327-333-03 (vial)
NDC: 62327-333-43 (carton)Store at 20°C - 25°C (68°F - 77°F); excursions permitted between 15°C - 30°C (59°F - 86°F) [see USP Controlled Room Temperature]. Protect against sunlight.
-
17. PATIENT COUNSELLING INFORMATION
- Advise patients to notify their healthcare provider if new or worsening pulmonary symptoms develop [see Warnings and Precautions (5.1)].
- Inform patients that the lead content in STERITALC can be harmful to a developing fetus. Advise females of reproductive potential to use effective contraception during treatment and for 5 months after the last dose of STERITALC [see Warnings and Precautions (5.3) and Use in Specific Populations (8.3)].
Manufactured for: Novatech SA
Z.I. Athélia III - 1058, Voie Antiope
13705 La Ciotat CEDEX
France
By: bess pro gmbh
Gustav-Krone-Strasse 7
14167 Berlin
Germany
NO128-03
- Principal Display Label, Steritalc 3g package label
- Principal Display Label, Steritalc 4g package label
- Principal Display Label, Steritalc 2g package label
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STERITALC 4G
talc powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62327-444 Route of Administration INTRAPLEURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TALC (UNII: 7SEV7J4R1U) (TALC - UNII:7SEV7J4R1U) TALC 4 g in 50 mL Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62327-444-44 4 in 1 BOX 08/01/2017 1 NDC: 62327-444-04 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205555 08/01/2017 STERITALC 2G
talc powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62327-222 Route of Administration INTRAPLEURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TALC (UNII: 7SEV7J4R1U) (TALC - UNII:7SEV7J4R1U) TALC 2 g in 50 mL Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62327-222-42 4 in 1 BOX 11/01/2017 1 NDC: 62327-222-02 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205555 11/01/2017 STERITALC 3G
talc powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62327-333 Route of Administration INTRAPLEURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TALC (UNII: 7SEV7J4R1U) (TALC - UNII:7SEV7J4R1U) TALC 3 g in 10 mL Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62327-333-43 4 in 1 BOX 11/01/2017 1 NDC: 62327-333-03 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA205555 11/01/2017 Labeler - Novatech SA (777211640) Registrant - Novatech SA (777211640) Establishment Name Address ID/FEI Business Operations Novatech SA 777211640 label(62327-444, 62327-333, 62327-222)
Trademark Results [Steritalc]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STERITALC 79008374 3093389 Live/Registered |
NOVATECH SA 2004-12-28 |
 STERITALC 75076198 2116833 Dead/Cancelled |
NOVATECH SA 1996-03-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.