SODIUM SULFACETAMIDE, SULFUR solution
SODIUM SULFACETAMIDE, SULFUR by
Drug Labeling and Warnings
SODIUM SULFACETAMIDE, SULFUR by is a Prescription medication manufactured, distributed, or labeled by Exact-Rx, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
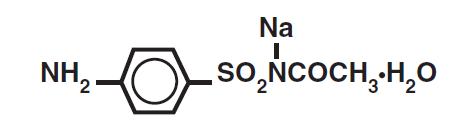
DESCRIPTION: Sodium sulfacetamide is a sulfonamide with antibacterial activity while sulfur acts as a keratolytic agent. Chemically sodium sulfacetamide is N-[(4-aminophenyl)sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
Each gram contains sodium sulfacetamide 10% (100 mg) and sulfur 5% (50 mg), purified water, disodium laureth sulfosuccinate and disodium lauryl sulfoacetate, PPG-hydroxyethyl coco isostearamide, sodium cocoyl isethionate, mineral oil, butylated hydroxytoluene, emulsifying wax, methyl paraben, propyl paraben, sodium thiosulfate, disodium EDTA and hydrochloric acid.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY: The most widely accepted mechanism of action of sulfonamides is the Woods-Fildes theory which is based on the fact that sulfonamides act as competitive antagonists to para-aminobenzoic acid (PABA), an essential component for bacterial growth. While absorption through intact skin has not been determined, sodium sulfacetamide is readily absorbed from the gastrointestinal tract when taken orally and excreted in the urine, largely unchanged. The biological half-life has variously been reported as 7 to 12.8 hours. The exact mode of action of sulfur in the treatment of acne is unknown, but it has been reported that it inhibits the growth of Propionibacterium acnes and the formation of free fatty acids.
- INDICATIONS & USAGE
- CONTRAINDICATIONS
-
WARNINGS
WARNINGS: Although it is rare, sensitivity to sodium sulfacetamide may occur. Therefore, caution and careful supervision should be observed when prescribing this drug for patients who may be prone to hypersensitivity to topical sulfonamides. Systemic toxic reactions such as agranulocytosis, acute hemolytic anemia, purpura hemorrhagica, drug fever, jaundice, and contact dermatitis indicate hypersensitivity to sulfonamides. Particular caution should be employed if areas of denuded or abraded skin are involved.
FOR EXTERNAL USE ONLY. Keep away from eyes.
KEEP OUT OF REACH OF CHILDREN.
In case of accidental ingestion contact a poison control center immediately. Keep container tightly closed.
-
WARNINGS AND PRECAUTIONS
PRECAUTIONS: General: If irritation develops, use of the product should be discontinued and appropriate therapy instituted. Patients should be carefully observed for possible local irritation or sensitization during long-term therapy. The object of this therapy is to achieve desquamation without irritation, but sodium sulfacetamide and sulfur can cause reddening and scaling of the epidermis. These side effects are not unusual in the treatment of acne vulgaris, but patients should be cautioned about the possibility.
- INFORMATION FOR PATIENTS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
-
PREGNANCY
Pregnancy: Category C. Animal reproduction studies have not been conducted with Sodium Sulfacetamide 10% & Sulfur 5% Cleanser. It is also not known whether Sodium Sulfacetamide 10% & Sulfur 5% Cleanser can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Sulfacetamide 10% & Sulfur 5% Cleanser should be given to a pregnant woman only if clearly needed.
-
NURSING MOTHERS
Nursing Mothers: It is not known whether sodium sulfacetamide is excreted in the human milk following topical use of Sodium Sulfacetamide 10% & Sulfur 5% Cleanser. However, small amounts of orally administered sulfonamides have milk. In view of this and because many drugs are excreted in human milk, caution should be exercised when Sodium Sulfacetamide 10% & Sulfur 5% Cleanser is administered to a nursing woman.
- PEDIATRIC USE
- ADVERSE REACTIONS
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: Wash affected areas once or twice daily, or as directed by your physician. Avoid contact with eyes or mucous membranes. Wet skin and liberally apply to areas to be cleansed, massage gently into skin for 10–20 seconds working into a full lather, rinse thoroughly and pat dry. If drying occurs, it may be controlled by rinsing cleanser off sooner or using less often.
-
HOW SUPPLIED
HOW SUPPLIED: Sodium Sulfacetamide 10% & Sulfur 5% Cleanser is available in a 6 oz (170 g) tube, NDC: 42808-0110-06 and 12 oz carton that contains (2) 6 oz tubes, NDC: 42808-0110-12.
- STORAGE AND HANDLING
-
PACKAGE LABEL - 12 oz (340 g)
For External Use Only
NDC: 42808-0110-12 Rx Only
Sodium Sulfacetamide
& Sulfur(Sodium Sulfacetamide 10% & Sulfur 5%)
10%/5%
CLEANSER
Exact-Rx.
INCORPORATEDContains 2 x 6 oz. tubes (170 g each)
Net Wt. 12 oz (340 g)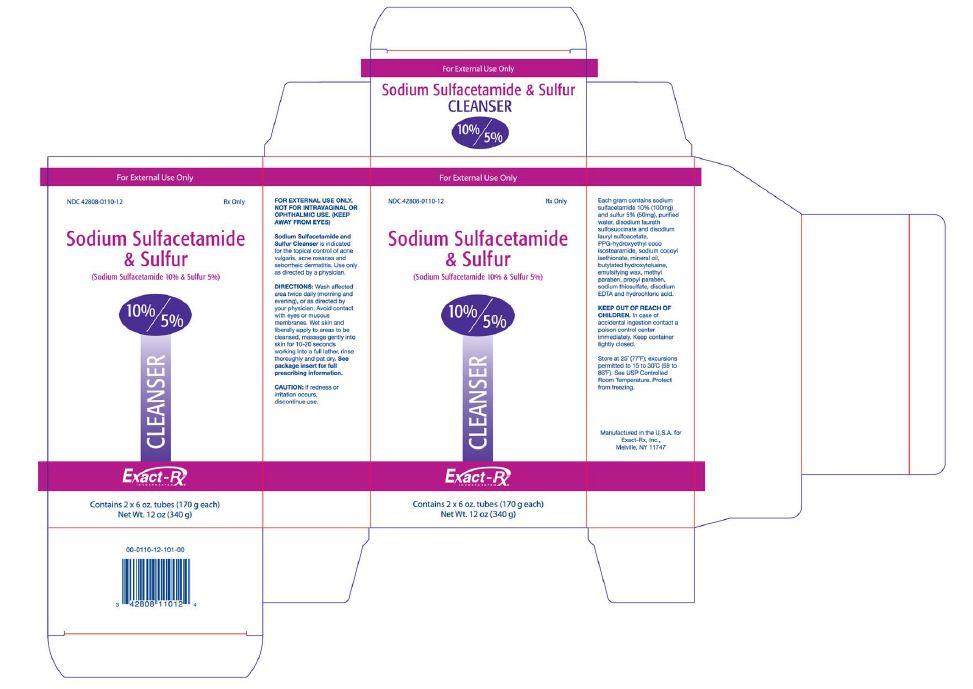
-
INGREDIENTS AND APPEARANCE
SODIUM SULFACETAMIDE, SULFUR
sodium sulfacetamide, sulfur solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42808-110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFACETAMIDE SODIUM (UNII: 4NRT660KJQ) (SULFACETAMIDE - UNII:4965G3J0F5) SULFACETAMIDE SODIUM 100 mg in 1 g SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 50 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) SODIUM LAURYL SULFOACETATE (UNII: D0Y70F2B9J) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) MINERAL OIL (UNII: T5L8T28FGP) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM THIOSULFATE (UNII: HX1032V43M) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) YELLOW WAX (UNII: 2ZA36H0S2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42808-110-06 170 g in 1 TUBE 2 NDC: 42808-110-12 2 in 1 CARTON 2 170 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/01/2011 Labeler - Exact-Rx, Inc. (137953498)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.