ALFENTANIL- alfentanil hydrochloride injection
Alfentanil by
Drug Labeling and Warnings
Alfentanil by is a Prescription medication manufactured, distributed, or labeled by Akorn, Inc., Akorn, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
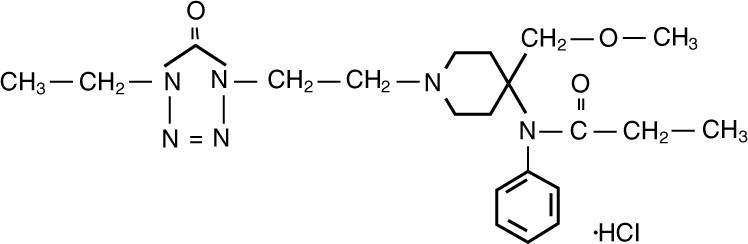
Alfentanil HCl Injection, USP is an opioid analgesic chemically designated as N-[1-[2-(4-ethyl-4,5-dihydro 5-oxo-1H-tetrazol-1-yl)ethyl]-4-(methoxymethyl)-4- piperidinyl]-N-phenylpropanamide monohydrochloride (1:1) with a molecular weight of 452.98 and an n-octanol:water partition coefficient of 128:1 at pH 7.4. The structural formula of Alfentanil hydrochloride is:
Alfentanil HCl Injection, USP is a sterile, non-pyrogenic, preservative free aqueous solution containing alfentanil hydrochloride equivalent to 500 μg per mL of alfentanil base for intravenous injection. The solution, which contains sodium chloride for isotonicity, has a pH range of 4 to 6. Each mL contains: Active: Alfentanil base 500 mcg. Inactives: Sodium Chloride 9 mg and Water for Injection q.s.
-
CLINICAL PHARMACOLOGY
Alfentanil is an opioid analgesic with a rapid onset of action.
At doses of 8 to 40 mcg/kg for surgical procedures lasting up to 30 minutes, alfentanil provides analgesic protection against hemodynamic responses to surgical stress with recovery times generally comparable to those seen with equipotent fentanyl dosages.
For longer procedures, doses of up to 75 mcg/kg attenuate hemodynamic responses to laryngoscopy, intubation and incision, with recovery time comparable to fentanyl. At doses of 50 to 75 mcg/kg followed by a continuous infusion of 0.5 to 3 mcg/kg/min, alfentanil attenuates the catecholamine response with more rapid recovery and reduced need for postoperative analgesics as compared to patients administered enflurane. At doses of 5 mcg/kg, alfentanil provides analgesia for the conscious but sedated patient. Based on patient response, doses higher than 5 mcg/kg may be needed. Elderly or debilitated patients may require lower doses. High intrasubject and intersubject variability in the pharmacokinetic disposition of alfentanil has been reported.
The pharmacokinetics of alfentanil can be described as a three-compartment model with sequential distribution half-lives of 1 and 14 minutes; and a terminal elimination half-life of 90 to 111 minutes (as compared to a terminal elimination half-life of approximately 475 minutes for fentanyl and approximately 265 minutes for sufentanil at doses of 250 mcg). The liver is the major site of biotransformation.
Alfentanil has an apparent volume of distribution of 0.4 to 1 L/kg, which is approximately one-fourth to one-tenth that of fentanyl, with an average plasma clearance of 5 mL/kg/min as compared to approximately 8 mL/kg/min for fentanyl.
Only 1% of the dose is excreted as unchanged drug; urinary excretion is the major route of elimination of metabolites. Plasma protein binding of alfentanil is approximately 92%.
In one study involving 15 patients administered alfentanil with nitrous oxide/oxygen, a narrow range of plasma alfentanil concentrations, approximately 310 to 340 ng/mL, was shown to provide adequate anesthesia for intra-abdominal surgery, while lower concentrations, approximately 190 ng/mL, blocked responses to skin closure. Plasma concentrations between 100 to 200 ng/mL provided adequate anesthesia for superficial surgery.
Alfentanil has an immediate onset of action. At dosages of approximately 105 mcg/kg, alfentanil produces hypnosis as determined by EEG patterns; an anesthetic ED90 of 182 mcg/kg for alfentanil in unpremedicated patients has been determined, based upon the ability to block response to placement of a nasopharyngeal airway. Based on clinical trials, induction dosage requirements range from 130 to 245 mcg/kg. For procedures lasting 30 to 60 minutes, loading dosages of up to 50 mcg/kg produce the hemodynamic response to endotracheal intubation and skin incision as comparable to those from fentanyl. A pre-intubation loading dose of 50 to 75 mcg/kg prior to a continuous infusion attenuates the response to laryngoscopy, intubation and incision. Subsequent administration of alfentanil infusion administered at a rate of 0.5 to 3 mcg/kg/min with nitrous oxide/oxygen attenuates sympathetic responses to surgical stress with more rapid recovery than enflurane.
Requirements for volatile inhalation anesthetics were reduced by thirty to fifty percent during the first 60 minutes of maintenance in patients administered anesthetic doses (above 130 mcg/kg) of alfentanil as compared to patients given doses of 4 to 5 mg/kg thiopental for anesthetic induction. At anesthetic induction dosages, alfentanil provides a deep level of anesthesia during the first hour of anesthetic maintenance and provides attenuation of the hemodynamic response during intubation and incision.
Following an anesthetic induction dose of alfentanil, requirements for alfentanil infusion are reduced by 30 to 50% for the first hour of maintenance.
Patients with compromised liver function and those over 65 years of age have been found to have reduced plasma clearance and extended terminal elimination for alfentanil, which may prolong postoperative recovery. Repeated or continuous administration of alfentanil produces increasing plasma concentrations and an accumulation of the drug, particularly in patients with reduced plasma clearance.
Bradycardia may be seen in patients administered alfentanil. The incidence and degree of bradycardia may be more pronounced when alfentanil is administered in conjunction with non-vagolytic neuromuscular blocking agents or in the absence of anticholinergic agents such as atropine.
Administration of intravenous diazepam immediately prior to or following high doses of alfentanil has been shown to produce decreases in blood pressure that may be secondary to vasodilation; recovery may also be prolonged.
Patients administered doses up to 200 mcg/kg of alfentanil have shown no significant increase in histamine levels and no clinical evidence of histamine release.
Skeletal muscle rigidity is related to the dose and speed of administration of alfentanil. Muscular rigidity will occur with an immediate onset following anesthetic induction dosages. Preventative measures (see WARNINGS) may reduce the rate and severity.
The duration and degree of respiratory depression and increased airway resistance usually increase with dose, but have also been observed at lower doses. Although higher doses may produce apnea and a longer duration of respiratory depression, apnea may also occur at low doses.
During monitored anesthesia care (MAC), attention must be given to the respiratory effects of alfentanil. Decreased oxygen saturation, apnea, decreased respiratory rate, and upper airway obstruction can occur. (See WARNINGS)
-
INDICATIONS AND USAGE
Alfentanil HCl injection is indicated:
- as an analgesic adjunct given in incremental doses in the maintenance of anesthesia with barbiturate/nitrous oxide/oxygen.
- as an analgesic administered by continuous infusion with nitrous oxide/oxygen in the maintenance of general anesthesia.
- as a primary anesthetic agent for the induction of anesthesia in patients undergoing general surgery in which endotracheal intubation and mechanical ventilation are required.
- as the analgesic component for monitored anesthesia care (MAC).
SEE DOSAGE CHART FOR MORE COMPLETE INFORMATION ON THE USE OF ALFENTANIL HCl INJECTION.
- CONTRAINDICATIONS
-
WARNINGS
ALFENTANIL SHOULD BE ADMINISTERED ONLY BY PERSONS SPECIFICALLY TRAINED IN THE USE OF INTRAVENOUS AND GENERAL ANESTHETIC AGENTS AND IN THE MANAGEMENT OF RESPIRATORY EFFECTS OF POTENT OPIOIDS.
AN OPIOID ANTAGONIST, RESUSCITATIVE AND INTUBATION EQUIPMENT AND OXYGEN SHOULD BE READILY AVAILABLE.
BECAUSE OF THE POSSIBILITY OF DELAYED RESPIRATORY DEPRESSION, MONITORING OF THE PATIENT MUST CONTINUE WELL AFTER SURGERY.
Alfentanil administered in initial dosages up to 20 mcg/kg may cause skeletal muscle rigidity, particularly of the truncal muscles. The incidence and severity of muscle rigidity is usually dose-related. Administration of alfentanil at anesthetic induction dosages (above 130 mcg/kg) will consistently produce muscular rigidity with an immediate onset. The onset of muscular rigidity occurs earlier than with other opioids. Alfentanil may produce muscular rigidity that involves all skeletal muscles, including those of the neck and extremities. The incidence may be reduced by: 1) routine methods of administration of neuromuscular blocking agents for balanced opioid anesthesia; 2) administration of up to 1/4 of the full paralyzing dose of a neuromuscular blocking agent just prior to administration of alfentanil at dosages up to 130 mcg/kg; following loss of consciousness, a full paralyzing dose of a neuromuscular blocking agent should be administered; or 3) simultaneous administration of alfentanil and a full paralyzing dose of a neuromuscular blocking agent when alfentanil is used in rapidly administered anesthetic dosages (above 130 mcg/kg).
The neuromuscular blocking agent used should be appropriate for the patient's cardiovascular status. Adequate facilities should be available for postoperative monitoring and ventilation of patients administered alfentanil. It is essential that these facilities be fully equipped to handle all degrees of respiratory depression.
PATIENTS RECEIVING MONITORED ANESTHESIA CARE (MAC) SHOULD BE CONTINUOUSLY MONITORED BY PERSONS NOT INVOLVED IN THE CONDUCT OF THE SURGICAL OR DIAGNOSTIC PROCEDURE; OXYGEN SUPPLEMENTATION SHOULD BE IMMEDIATELY AVAILABLE AND PROVIDED WHERE CLINICALLY INDICATED; OXYGEN SATURATION SHOULD BE CONTINUOUSLY MONITORED; THE PATIENT SHOULD BE OBSERVED FOR EARLY SIGNS OF HYPOTENSION, APNEA, UPPER AIRWAY OBSTRUCTION AND/OR OXYGEN DESATURATION.
Severe and unpredictable potentiation of monoamine oxidase (MAO) inhibitors has been reported for other opioid analgesics, and rarely with alfentanil. Therefore when alfentanil is administered to patients who have received MAO inhibitors within 14 days, appropriate monitoring and ready availability of vasodilators and betablockers for the treatment of hypertension is recommended.
-
PRECAUTIONS
DELAYED RESPIRATORY DEPRESSION, RESPIRATORY ARREST, BRADYCARDIA, ASYSTOLE, ARRHYTHMIAS AND HYPOTENSION HAVE ALSO BEEN REPORTED. THEREFORE, VITAL SIGNS MUST BE MONITORED CONTINUOUSLY.
General: The initial dose of alfentanil should be appropriately reduced in elderly and debilitated patients. The effect of the initial dose should be considered in determining supplemental doses. In obese patients (more than 20% above ideal total body weight), the dosage of alfentanil should be determined on the basis of lean body weight.
In one clinical trial, the dose of alfentanil required to produce anesthesia, as determined by appearance of delta waves in EEG, was 40% lower in geriatric patients than that needed in healthy young patients.
In patients with compromised liver function and in geriatric patients, the plasma clearance of alfentanil may be reduced and postoperative recovery may be prolonged.
Induction doses of alfentanil should be administered slowly (over three minutes). Administration may produce loss of vascular tone and hypotension. Consideration should be given to fluid replacement prior to induction.
Diazepam administered immediately prior to or in conjunction with high doses of alfentanil may produce vasodilation, hypotension and result in delayed recovery.
Bradycardia produced by alfentanil may be treated with atropine. Severe bradycardia and asystole have been successfully treated with atropine and conventional resuscitative methods.
The hemodynamic effects of a particular muscle relaxant and the degree of skeletal muscle relaxation required should be considered in the selection of a neuromuscular blocking agent.
Following an anesthetic induction dose of alfentanil, requirements for volatile inhalation anesthetics or alfentanil infusion are reduced by 30 to 50% for the first hour of maintenance.
Alfentanil infusions should be discontinued at least 10 to 15 minutes prior to the end of surgery during general anesthesia. During administration of alfentanil for Monitored Anesthesia Care (MAC), infusions may be continued to the end of the procedure.
Respiratory depression caused by opioid analgesics can be reversed by opioid antagonists such as naloxone. Because the duration of respiratory depression produced by alfentanil may last longer than the duration of the opioid antagonist action, appropriate surveillance should be maintained. As with all potent opioids, profound analgesia is accompanied by respiratory depression and diminished sensitivity to CO2 stimulation which may persist into or recur in the postoperative period. Intraoperative hyperventilation may further alter postoperative response to CO2. Appropriate postoperative monitoring should be employed, particularly after infusions and large doses of alfentanil, to ensure that adequate spontaneous breathing is established and maintained in the absence of stimulation prior to discharging the patient from the recovery area.
Head Injuries: Alfentanil should be used with caution in patients with head injury or increased intracranial pressure, due to the increased risk of respiratory depression. As with all opioids, alfentanil may obscure the clinical course of patients with head injuries and should be used only if clinically indicated.
Impaired Respiration: Alfentanil should be used with caution in patients with pulmonary disease, decreased respiratory reserve or potentially compromised respiration. In such patients, opioids may additionally decrease respiratory drive and increase airway resistance. During anesthesia, this can be managed by assisted or controlled respiration.
Drug Interactions: Both the magnitude and duration of central nervous system and cardiovascular effects may be enhanced when alfentanil is administered in combination with other CNS depressants such as barbiturates, tranquilizers, opioids, or inhalation general anesthetics. Postoperative respiratory depression may be enhanced or prolonged by these agents. In such cases of combined treatment, the dose of one or both agents should be reduced. Limited clinical experience indicates that requirements for volatile inhalation anesthetics are reduced by 30 to 50% for the first sixty (60) minutes following alfentanil induction. The concomitant use of erythromycin with alfentanil can significantly inhibit alfentanil clearance and may increase the risk of prolonged or delayed respiratory depression.
Cimetidine reduces the clearance of alfentanil. Therefore smaller alfentanil doses will be required with prolonged administration and the duration of action of alfentanil may be extended.
Perioperative administration of drugs affecting hepatic blood flow or enzyme function may reduce plasma clearance and prolong recovery.
Carcinogenesis, Mutagenesis and Impairment of Fertility: No long-term animal studies of alfentanil have been performed to evaluate carcinogenic potential. No structural chromosome mutations were produced in the in vivo micronucleus test in female rats at single intravenous doses of alfentanil as high as 20 mg/kg body weight (approximately 40 times the upper human dose), equivalent to a dose of 103 mg/m2 body surface area. No dominant lethal mutations were produced in the in vivo dominant lethal test in male and female mice at the maximum intravenous dose of 20 mg/kg (60 mg/m2). No mutagenic activity was revealed in the in vitro Ames Salmonella typhimurium test, with and without metabolic activation.
Pregnancy Category C: Alfentanil has been shown to have an embryocidal effect in rats and rabbits when given in doses 2.5 times the upper human dose for a period of 10 days to over 30 days. These effects could have been due to maternal toxicity (decreased food consumption with increased mortality) following prolonged administration of the drug.
No evidence of teratogenic effects has been observed after administration of alfentanil in rats or rabbits.
There are no adequate and well-controlled studies in pregnant women. Alfentanil should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Labor and Delivery: There are insufficient data to support the use of alfentanil in labor and delivery. Placental transfer of the drug has been reported; therefore, use in labor and delivery is not recommended.
Nursing Mothers: In one study of nine women undergoing postpartum tubal ligation, significant levels of alfentanil were detected in colostrum four hours after administration of 60 mcg/kg of alfentanil, with no detectable levels present after 28 hours. Caution should be exercised when alfentanil is administered to a nursing woman.
-
ADVERSE REACTIONS
The most common adverse reactions of opioids are respiratory depression and skeletal muscle rigidity, particularly of the truncal muscles. Alfentanil may produce muscular rigidity that involves the skeletal muscles of the neck and extremities. See CLINICAL PHARMACOLOGY, WARNINGS, and PRECAUTIONS on the management of respiratory depression and skeletal muscle rigidity.
The adverse experience profile from 696 patients receiving alfentanil for Monitored Anesthesia Care (MAC) is similar to the profile established with alfentanil during general anesthesia. Respiratory events reported during MAC included hypoxia, apnea, and bradypnea. Other adverse events reported by patients receiving alfentanil for MAC, in order of decreasing frequency, were nausea, hypotension, vomiting, pruritus, confusion, somnolence and agitation.
The following adverse reaction information is derived from controlled and open clinical trials in 785 patients who received intravenous alfentanil during induction and maintenance of general anesthesia. The controlled trials included treatment comparisons with fentanyl, thiopental sodium, enflurane, saline placebo and halothane. The incidence of certain side effects is influenced by the type of use, e.g., chest wall rigidity has a higher reported incidence in clinical trials of alfentanil induction, and by the type of surgery, e.g., nausea and vomiting have a higher reported incidence in patients undergoing gynecologic surgery. The overall reports of nausea and vomiting with alfentanil were comparable to fentanyl.
Incidence Greater than 1% - Probably Causally Related (Derived from clinical trials)
*Incidence 3% to 9%
All others 1% to 3%
Gastrointestinal: nausea (28%), vomiting (18%) Cardiovascular: arrhythmia, bradycardia (14%), hypertension (18%), hypotension (10%), tachycardia (12%) Musculoskeletal: chest wall rigidity (17%), skeletal muscle movements* Respiratory: apnea*, postoperative respiratory depression Central Nervous System: blurred vision, dizziness*, sleepiness/postoperative sedation Incidence Less than 1% - Probably Causally Related (Derived from clinical trials)
Adverse events reported in post-marketing surveillance, not seen in clinical trials, are italicized.
*Incidence 0.3% to 1%
Body as a whole: anaphylaxis Central Nervous System: headache*, myoclonic movements, postoperative confusion*, postoperative euphoria*, shivering* Dermatological: itching*, urticaria* Injection Site: pain* Musculoskeletal: skeletal muscle rigidity of neck and extremities Respiratory: bronchospasm, hypercarbia*, laryngospasm* -
DRUG ABUSE AND DEPENDENCE
Alfentanil is a Schedule II controlled drug substance that can produce drug dependence of the morphine type and therefore has the potential for being abused.
Opioid analgesics have been associated with abuse and dependence in health care providers and others with ready access to such drugs. Alfentanil should be handled accordingly.
-
OVERDOSAGE
Overdosage would be manifested by extension of the pharmacological actions of alfentanil (see CLINICAL PHARMACOLOGY) as with other potent opioid analgesics. No experience of overdosage with alfentanil was reported during clinical trials. The intravenous LD50 of alfentanil is 43 to 51 mg/kg in rats, 72 to 74 mg/kg in mice, 72 to 82 mg/kg in guinea pigs and 60 to 88 mg/kg in dogs. Intravenous administration of an opioid antagonist such as naloxone should be employed as a specific antidote to manage respiratory depression.
The duration of respiratory depression following overdosage with alfentanil may be longer than the duration of action of the opioid antagonist. Administration of an opioid antagonist should not preclude immediate establishment of a patent airway, administration of oxygen, and assisted or controlled ventilation as indicated for hypoventilation or apnea. If respiratory depression is associated with muscular rigidity, a neuromuscular blocking agent may be required to facilitate assisted or controlled ventilation. Intravenous fluids and vasoactive agents may be required to manage hemodynamic instability.
-
DOSAGE AND ADMINISTRATION
The dosage of Alfentanil HCl injection should be individualized and titrated to the desired effect in each patient according to body weight, physical status, underlying pathological condition, use of other drugs, and type and duration of surgical procedure and anesthesia. In obese patients (more than 20% above ideal total body weight), the dosage of Alfentanil HCl injection should be determined on the basis of lean body weight. The dose of Alfentanil HCl injection should be reduced in elderly or debilitated patients (see PRECAUTIONS).
Vital signs should be monitored routinely.
See Dosage Guidelines for the use of Alfentanil HCl injection: 1) by incremental injection as an analgesic adjunct to anesthesia with barbiturate/nitrous oxide/oxygen for short surgical procedures (expected duration of less than one hour); 2) by continuous infusion as a maintenance analgesic with nitrous oxide/oxygen for general surgical procedures; and 3) by intravenous injection in anesthetic doses for the induction of anesthesia for general surgical procedures with a minimum expected duration of 45 minutes; and 4) by intravenous injection as the analgesic component for monitored anesthesia care (MAC).
DOSAGE GUIDELINES
DOSAGE SHOULD BE INDIVIDUALIZED AND TITRATEDFOR USE DURING GENERAL ANESTHESIA SPONTANEOUSLY BREATHING/
ASSISTED VENTILATIONInduction of Analgesia: 8 to 20 mcg/kg
Maintenance of Analgesia: 3 to 5 mcg/kg q 5 to 20 min or 0.5 to 1 mcg/kg/min
Total dose: 8 to 40 mcg/kgASSISTED OR CONTROLLED
VENTILATION
Induction of Analgesia: 20 to 50 mcg/kg
Maintenance of Analgesia: 5 to 15 mcg/kg q 5 to 20 min
Total dose: Up to 75 mcg/kgIncremental Injection
(To attenuate response to laryngoscopy
and intubation)Continuous Infusion
(To provide attenuation of response to
intubation and incision)Infusion rates are variable and should be titrated to the desired clinical effect.
SEE INFUSION DOSAGE GUIDELINES BELOW.
Induction of Analgesia: 50 to 75 mcg/kg
Maintenance of Analgesia: 0.5 to 3 mcg/kg/min (Average rate 1 to 1.5 mcg/kg/min)
Total dose: Dependent on duration of procedureAnesthetic Induction Induction of Anesthesia: 130 to 245 mcg/kg
Maintenance of Anesthesia: 0.5 to 1.5 mcg/kg/min or general anesthetic
Total dose: Dependent on duration of procedure
At these doses, truncal rigidity should be expected and a muscle relaxant should be utilized.
Administer slowly (over 3 minutes).
Concentration of inhalation agents reduced by 30 to 50% for initial hour.MONITORED ANESTHESIA CARE
(MAC)
(For sedated and responsive
spontaneously breathing patients)Induction of MAC: 3 to 8 mcg/kg
Maintenance of MAC: 3 to 5 mcg/kg q 5 to 20 min or 0.25 to 1 mcg/kg/min
Total dose: 3 to 40 mcg/kgINFUSION DOSAGE Continuous Infusion: 0.5 to 3 mcg/kg/min administered with nitrous oxide/oxygen in patients undergoing general surgery. Following an anesthetic induction dose of Alfentanil HCl injection, infusion rate requirements are reduced by 30 to 50% for the first hour of maintenance.
Changes in vital signs that indicate a response to surgical stress or lightening of anesthesia may be controlled by increasing the alfentanil to a maximum of 4 mcg/kg/min and/or administration of bolus doses of 7 mcg/kg. If changes are not controlled after three bolus doses given over a five minute period, a barbiturate, vasodilator, and/or inhalation agent should be used. Infusion rates should always be adjusted downward in the absence of these signs until there is some response to surgical stimulation.
Rather than an increase in infusion rate, 7 mcg/kg bolus doses of Alfentanil HCl injection or a potent inhalation agent should be administered in response to signs of lightening of anesthesia within the last 15 minutes of surgery. Alfentanil HCl injection infusion should be discontinued at least 10 to 15 minutes prior to the end of surgery.Usage in Children: Clinical data to support the use of Alfentanil HCl injection in patients under 12 years of age are not presently available. Therefore, such use is not recommended.
Premedication: The selection of preanesthetic medications should be based upon the needs of the individual patient.
Neuromuscular Blocking Agents: The neuromuscular blocking agent selected should be compatible with the patient's condition, taking into account the hemodynamic effects of a particular muscle relaxant and the degree of skeletal muscle relaxation required (see CLINICAL PHARMACOLOGY, WARNINGS and PRECAUTIONS sections).
In patients administered anesthetic (induction) dosages of Alfentanil HCl injection, it is essential that qualified personnel and adequate facilities are available for the management of intraoperative and postoperative respiratory depression.
Also see WARNINGS and PRECAUTIONS sections.
For purposes of administering small volumes of Alfentanil HCl injection accurately, the use of a tuberculin syringe or equivalent is recommended.
The physical and chemical compatibility of Alfentanil HCl injection have been demonstrated in solution with normal saline, 5% dextrose in normal saline, 5% dextrose in water and Lactated Ringers. Clinical studies of Alfentanil HCl injection infusion have been conducted with Alfentanil HCl injection diluted to a concentration range of 25 mcg/mL to 80 mcg/mL.
As an example of the preparation of Alfentanil HCl injection for infusion, 20 mL of Alfentanil HCl injection added to 230 mL of diluent provides 40 mcg/mL solution of Alfentanil HCl injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- SAFETY AND HANDLING
-
HOW SUPPLIED
Alfentanil HCl Injection, USP for intravenous use. Each mL contains: Active: Alfentanil base 500 mcg. Inactives: Sodium Chloride 9 mg and WFI q.s. Alfentanil HCl Injection, USP is available as:
NDC: 17478-841-02, 2 mL Ampule in packages of 10
NDC: 17478-841-05, 5 mL Ampule in packages of 10
NDC: 17478-841-10, 10 mL Ampule in packages of 5
NDC: 17478-841-20, 20 mL Ampule in packages of 5U.S. Patent No. 4,167,574
May 1995, November 1995PREMIERProRx®
Manufactured by: Akorn, Inc.
Lake Forest, IL 60045
PremierProRx® is a registered trademark of Premier Inc., used under license.PAFA0N Rev. 10/15
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 17478-841-02 2 mL Ampule
Alfentanil HCl
Injection, USP CII
500 mcg/mL
Alfentanil base
Rx only
May be habit forming.
Premier Logo
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 17478-841-02 10 Ampules (2 mL each)
Alfentanil HCl
Injection, USP CII
500 mcg/mL
FOR INTRAVENOUS USE
Premier Logo
Rx only
-
INGREDIENTS AND APPEARANCE
ALFENTANIL
alfentanil hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 17478-841 Route of Administration INTRAVENOUS DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Alfentanil Hydrochloride (UNII: 11S92G0TIW) (Alfentanil - UNII:1N74HM2BS7) Alfentanil 500 ug in 1 mL Inactive Ingredients Ingredient Name Strength Sodium Chloride (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17478-841-02 10 in 1 CARTON 07/19/2013 1 2 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 17478-841-05 10 in 1 CARTON 07/19/2013 2 5 mL in 1 AMPULE; Type 0: Not a Combination Product 3 NDC: 17478-841-10 5 in 1 CARTON 07/19/2013 3 10 mL in 1 AMPULE; Type 0: Not a Combination Product 4 NDC: 17478-841-20 5 in 1 CARTON 07/19/2013 4 20 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019353 07/19/2013 Labeler - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc 063434679 PACK(17478-841) , LABEL(17478-841) Establishment Name Address ID/FEI Business Operations Akorn, Inc. 155135783 MANUFACTURE(17478-841) , ANALYSIS(17478-841) , STERILIZE(17478-841)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.