MYALEPT- metreleptin injection, powder, lyophilized, for solution
Myalept by
Drug Labeling and Warnings
Myalept by is a Prescription medication manufactured, distributed, or labeled by Amryt Pharmaceuticals DAC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MYALEPT safely and effectively. See full prescribing information for MYALEPT.
MYALEPT® (metreleptin) for injection for subcutaneous use
Initial U.S. Approval: 2014WARNING: RISK OF ANTI-METRELEPTIN ANTIBODIES WITH NEUTRALIZING ACTIVITY AND RISK OF LYMPHOMA
See full prescribing information for complete boxed warning.
Anti-metreleptin antibodies with neutralizing activity have been identified in patients treated with MYALEPT. The consequences are not well characterized but could include inhibition of endogenous leptin action and loss of MYALEPT efficacy. Worsening metabolic control and/or severe infection have been reported. Test for anti-metreleptin antibodies with neutralizing activity in patients with severe infections or loss of efficacy during MYALEPT treatment. Contact Aegerion Pharmaceuticals, Inc. at 1-866-216-1526 for neutralizing antibody testing. (4.1), (5.1)
T-cell lymphoma has been reported in patients with acquired generalized lipodystrophy, both treated and not treated with MYALEPT. Carefully consider the benefits and risks of treatment with MYALEPT in patients with significant hematologic abnormalities and/or acquired generalized lipodystrophy. (5.2)
MYALEPT is available only through a restricted program called the MYALEPT REMS PROGRAM. (5.3)
INDICATIONS AND USAGE
MYALEPT is a leptin analog indicated as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy. (1)
Limitations of Use
- The safety and effectiveness of MYALEPT for the treatment of complications of partial lipodystrophy have not been established. (1)
- The safety and effectiveness of MYALEPT for the treatment of liver disease, including nonalcoholic steatohepatitis (NASH), have not been established. (1)
- MYALEPT is not indicated for use in patients with HIV-related lipodystrophy. (1)
- MYALEPT is not indicated for use in patients with metabolic disease, without concurrent evidence of generalized lipodystrophy. (1)
DOSAGE AND ADMINISTRATION
Administer as a subcutaneous injection once daily after the lyophilized cake is reconstituted with Bacteriostatic Water for Injection (BWFI) or preservative-free sterile Water for Injection (WFI). (2.1)
The recommended daily dosages are:
- Body weight 40 kg or less: starting dose 0.06 mg/kg/day, increase or decrease by 0.02 mg/kg to a maximum daily dose of 0.13 mg/kg. (2.1)
- Males greater than 40 kg body weight: starting dose 2.5 mg/day, increase or decrease by 1.25 mg to 2.5 mg/day to a maximum dose of 10 mg/day. (2.1)
- Females greater than 40 kg body weight: starting dose 5 mg/day, increase or decrease by 1.25 mg to 2.5 mg/day to a maximum dose of 10 mg/day. (2.1)
DOSAGE FORMS AND STRENGTHS
MYALEPT is supplied as a sterile, white, solid, lyophilized cake of 11.3 mg metreleptin per vial to deliver 5 mg per mL when reconstituted in 2.2 mL of BWFI or WFI. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Anti-metreleptin antibodies with neutralizing activity: Could inhibit endogenous leptin action and/or result in loss of MYALEPT efficacy. Test for neutralizing antibodies in patients with severe infections or loss of efficacy during MYALEPT treatment. (5.1)
- T-cell lymphoma: Carefully consider benefits and risks of treatment with MYALEPT in patients with significant hematologic abnormalities and/or acquired generalized lipodystrophy. (5.2)
- Hypoglycemia: A dose adjustment, including possible large reductions, of insulin or insulin secretagogue may be necessary. Closely monitor blood glucose in patients on concomitant insulin or insulin secretagogue therapy. (5.4)
- Autoimmunity: Autoimmune disorder progression has been observed in patients treated with MYALEPT. Carefully consider benefits and risks of MYALEPT treatment in patients with autoimmune disease. (5.5)
- Hypersensitivity: Hypersensitivity reactions (e.g., anaphylaxis, urticaria or generalized rash) have been reported. Patient should promptly seek medical advice regarding suspected reactions. (5.6)
- Benzyl Alcohol Toxicity: Preservative-free sterile WFI recommended for neonates and infants. (5.7)
ADVERSE REACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISK OF ANTI-METRELEPTIN ANTIBODIES WITH NEUTRALIZING ACTIVITY AND RISK OF LYMPHOMA
1 INDICATIONS AND USAGE
1.1 Patients with Generalized Lipodystrophy
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 MYALEPT Preparation and Storage
2.3 Administration Instructions
2.4 Dosage Adjustments of Medications Known to Cause Hypoglycemia
2.5 Discontinuation in Patients at Risk for Pancreatitis
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 General Obesity
4.2 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Risk for Development of Antibodies that Neutralize Endogenous Leptin and/or MYALEPT
5.2 Lymphoma
5.3 MYALEPT REMS Program
5.4 Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
5.5 Autoimmunity
5.6 Hypersensitivity
5.7 Benzyl Alcohol Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Open-Label, Single-Arm Study
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK OF ANTI-METRELEPTIN ANTIBODIES WITH NEUTRALIZING ACTIVITY AND RISK OF LYMPHOMA
Anti-metreleptin antibodies with neutralizing activity have been identified in patients treated with MYALEPT. The consequences of these neutralizing antibodies are not well characterized but could include inhibition of endogenous leptin action and/or loss of MYALEPT efficacy. Severe infection and/or worsening metabolic control have been reported. Test for anti-metreleptin antibodies with neutralizing activity in patients who develop severe infections or show signs suspicious for loss of MYALEPT efficacy during treatment. Contact Aegerion Pharmaceuticals, Inc. at 1-866-216-1526 for neutralizing antibody testing of clinical samples [see Contraindications (4.1) and Warnings and Precautions (5.1)].
T-cell lymphoma has been reported in patients with acquired generalized lipodystrophy, both treated and not treated with MYALEPT. Carefully consider the benefits and risks of treatment with MYALEPT in patients with significant hematologic abnormalities and/or acquired generalized lipodystrophy [see Warnings and Precautions (5.2)].
Because of these risks associated with the development of anti-metreleptin antibodies that neutralize endogenous leptin and/or MYALEPT and the risk for lymphoma, MYALEPT is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the MYALEPT REMS PROGRAM [see Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGE
1.1 Patients with Generalized Lipodystrophy
MYALEPT (metreleptin) for injection is indicated as an adjunct to diet as replacement therapy to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy.
Limitations of Use
- The safety and effectiveness of MYALEPT for the treatment of complications of partial lipodystrophy have not been established.
- The safety and effectiveness of MYALEPT for the treatment of liver disease, including nonalcoholic steatohepatitis (NASH), have not been established.
- MYALEPT is not indicated for use in patients with HIV-related lipodystrophy.
- MYALEPT is not indicated for use in patients with metabolic disease, including diabetes mellitus and hypertriglyceridemia, without concurrent evidence of congenital or acquired generalized lipodystrophy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
See Table 1 for the recommended daily dose and maximum recommended daily dose in adults and pediatric patients.
Based on clinical response (e.g., inadequate metabolic control) or other considerations (e.g., tolerability issues, excessive weight loss [especially in pediatric patients]), MYALEPT dosage may be decreased or increased to the maximum dosage listed in Table 1.
Table 1: MYALEPT Recommended Dosage Baseline weight Starting daily dose
(injection volume)Dose adjustments
(injection volume)Maximum daily dose
(injection volume)Less than or equal to 40 kg
(males and females)0.06 mg/kg
(0.012 mL/kg)0.02 mg/kg
(0.004 mL/kg)0.13 mg/kg
(0.026 mL/kg)Males greater than 40 kg
2.5 mg
(0.5 mL)1.25 mg (0.25 mL) to
2.5 mg (0.5 mL)10 mg
(2 mL)Females greater than 40 kg
5 mg
(1 mL)1.25 mg (0.25 mL) to
2.5 mg (0.5 mL)10 mg
(2 mL)In pediatric patients, small volumes for administration can result in medication errors when measured incorrectly [see Dosage and Administration (2.3), Adverse Reactions (6.3)]. Table 2 provides example doses and volumes by weight. For patients using insulin syringes, the volume conversion is 100 Units/mL.
Table 2: Example Dosing Chart for Patients Less than or Equal to 40 kg Weight Starting Dose Dose Adjustment Maximum Dose 5 kg 0.30 mg (0.06 mL or 6 Units) 0.10 mg (0.02 mL or 2 Units) 0.65 mg (0.13 mL or 13 Units) 10 kg 0.60 mg (0.12 mL or 12 Units) 0.20 mg (0.04 mL or 4 Units) 1.3 mg (0.26 mL or 26 Units) 15 kg 0.90 mg (0.18 mL or 18 Units) 0.30 mg (0.06 mL or 6 Units) 1.95 mg (0.39 mL or 39 Units) 20 kg 1.2 mg (0.24 mL or 24 Units) 0.40 mg (0.08 mL or 8 Units) 2.6 mg (0.52 mL or 52 Units) 25 kg 1.5 mg (0.3 mL or 30 Units) 0.50 mg (0.1 mL or 10 Units) 3.25 mg (0.65 mL or 65 Units) 30 kg 1.8 mg (0.36 mL or 36 Units) 0.60 mg (0.12 mL or 12 Units) 3.9 mg (0.78 mL or 78 Units) 35 kg 2.1 mg (0.42 mL or 42 Units) 0.70 mg (0.14 mL or 14 Units) 4.55 mg (0.91 mL or 91 Units) 40 kg 2.4 mg (0.48 mL or 48 Units) 0.80 mg (0.16 mL or 16 Units) 5.2 mg (1.03 mL or 103 Units) MYALEPT should be administered once daily at the same time every day. MYALEPT can be administered any time of day without regard to the timing of meals.
Instruct patients that if a dose is missed, administer the dose as soon as noticed, and resume the normal dosing schedule the next day.
2.2 MYALEPT Preparation and Storage
Healthcare practitioners should provide proper training to patients and caregivers regarding how to prepare and administer the correct dose of MYALEPT prior to self-use. The patients and caregivers should prepare and administer the first dose of MYALEPT under the supervision of a qualified healthcare professional.
Instruct patients to store the vials of lyophilized powder in their carton in the refrigerator as soon as received [see How Supplied/Storage and Handling (16.2)].
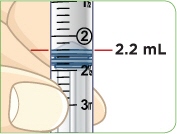
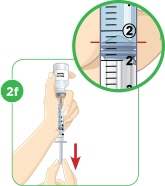
MYALEPT can be reconstituted aseptically with 2.2 mL of sterile Bacteriostatic Water for Injection (BWFI), USP (0.9% benzyl alcohol), or with 2.2 mL of sterile Water for Injection (WFI).
When reconstituted in BWFI, MYALEPT solution can be used within 3 days when stored in the refrigerator between 36°F and 46°F (2°C and 8°C) and protected from light [see How Supplied/Storage and Handling (16.2)]. Discard unused reconstituted solution after 3 days. Attach the supplied sticker to the vial and enter the discard date.
For use in neonates and infants, reconstitute with preservative-free sterile WFI [see Warnings and Precautions (5.7) and Use in Specific Populations (8.4)]. When reconstituted in sterile WFI, MYALEPT should be administered immediately. Unused reconstituted solution cannot be saved for later use and should be discarded.
Reconstitution of the Lyophilized Powder
Instruct patients to follow the directions below for reconstitution of the lyophilized powder:
- a) Remove the vial containing the MYALEPT lyophilized powder from the refrigerator and allow the vial to warm to room temperature prior to use.
- b) Visually inspect the vial containing MYALEPT. The cake of lyophilized powder should be intact and white in color.
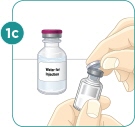
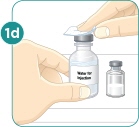
- c) Using a 3-mL syringe with a 22-gauge or smaller diameter needle withdraw 2.2 mL of sterile Bacteriostatic Water for Injection (BWFI) or preservative-free sterile Water for Injection (WFI). Do not reconstitute MYALEPT with other diluents.
- d) Inject the BWFI or WFI into the vial containing the lyophilized powder of MYALEPT, slowly injecting down the side of the vial. It is normal for some bubbles to form.
- e) Remove the needle and syringe from the vial and gently swirl the contents to reconstitute. Do not shake or vigorously agitate. When properly mixed, the MYALEPT reconstituted solution should be clear and free of clumps or dry powder, bubbles or foam. Do not use the solution if discolored or cloudy, or if particulate matter remains.
- f)
Regarding the compatibility of MYALEPT reconstituted solution with other solutions:
- Do not mix with, or transfer into, the contents of another vial of MYALEPT.
- Do not add other medications, including insulin. Use a separate syringe for insulin injections.
See the MYALEPT Instructions for Use for complete administration instructions. The instructions can also be found at www.myalept.com.
2.3 Administration Instructions
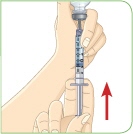
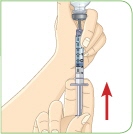
Healthcare practitioners should instruct patients and caregivers on the proper subcutaneous injection technique with care to avoid intramuscular injection in patients with minimal subcutaneous adipose tissue. Never administer MYALEPT intravenously or intramuscularly.
Instruct patients to follow the recommended injection technique:
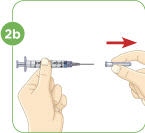
- a) Using a 1-mL or smaller syringe with a needle appropriate for subcutaneous injection, withdraw the prescribed dose of MYALEPT reconstituted solution.
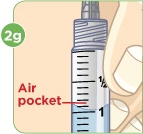
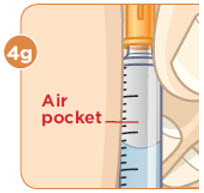
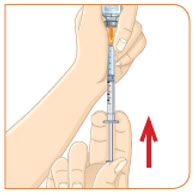
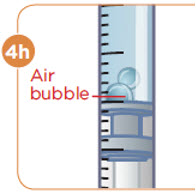
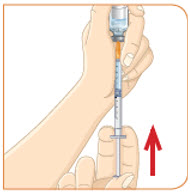
- b) Remove any large air pockets or large bubbles from the filled syringe prior to administration. Some small bubbles may remain in the syringe.
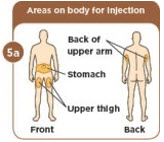
- c) Administer MYALEPT into the subcutaneous tissue of the abdomen, thigh or upper arm. Advise patients to use a different injection site each day when injecting in the same region. After choosing an injection site, pinch the skin and at a 45-degree angle, inject the MYALEPT reconstituted solution subcutaneously. Avoid intramuscular injection, especially in patients with minimal subcutaneous adipose tissue.
- d) Doses exceeding 1 mL can be administered as two injections (the total daily dose divided equally) to minimize potential injection-site discomfort due to injection volume. When dividing doses due to volume, doses can be administered one after the other.
- e) In pediatric patients, small volumes for administration can result in medication errors when measured incorrectly. Smaller syringe sizes may be more appropriate for pediatric patients weighing less than or equal to 25 kg. Ensure the proper size syringe is selected. Table 2 provides example doses and volumes by weight [see Dosage and Administration (2.1), Adverse Reactions (6.3)].
Do not mix MYALEPT with insulin. Use a separate syringe for each medication. If MYALEPT and insulin are administered at the same time of day, they may be injected in the same body area using two different injection sites.
See the MYALEPT Instructions for Use for complete administration instructions. The instructions can also be found at www.myalept.com.
2.4 Dosage Adjustments of Medications Known to Cause Hypoglycemia
Dosage adjustments, including possible large reductions, of insulin or insulin secretagogue (e.g., sulfonylurea) may be necessary in some patients to minimize the risk of hypoglycemia [see Warnings and Precautions (5.4) and Adverse Reactions (6.1)]. Closely monitor blood glucose in patients on concomitant insulin therapy, especially those on high doses, or insulin secretagogue (e.g., sulfonylurea) when treating with MYALEPT.
2.5 Discontinuation in Patients at Risk for Pancreatitis
When discontinuing MYALEPT therapy in patients with risk factors for pancreatitis (e.g., history of pancreatitis, severe hypertriglyceridemia), tapering of the dose over a one-week period is recommended. During tapering, monitor triglyceride levels and consider initiating or adjusting the dose of lipid-lowering medications as needed. Signs and/or symptoms consistent with pancreatitis should prompt an appropriate clinical evaluation.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 General Obesity
MYALEPT is contraindicated in patients with general obesity not associated with congenital leptin deficiency. MYALEPT has not been shown to be effective in treating general obesity, and the development of anti-metreleptin antibodies with neutralizing activity has been reported in obese patients treated with MYALEPT [see Warnings and Precautions (5.1)].
4.2 Hypersensitivity
MYALEPT is contraindicated in patients with prior severe hypersensitivity reactions to metreleptin or to any of the product components. Known hypersensitivity reactions have included anaphylaxis, urticaria and generalized rash [see Warnings and Precautions (5.6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk for Development of Antibodies that Neutralize Endogenous Leptin and/or MYALEPT
Anti-metreleptin antibodies with in vitro neutralizing activity to leptin associated with adverse events consistent with loss of endogenous leptin activity and/or loss of efficacy have been identified in two patients with generalized lipodystrophy treated with MYALEPT (severe infections, increases in HbA1c and triglycerides), and in three patients without lipodystrophy who received MYALEPT in clinical studies (excessive weight gain, development of glucose intolerance or diabetes mellitus). The clinical implications associated with development of anti-metreleptin antibodies with neutralizing activity are not well characterized at this time due to the small number of reports. Test for anti-metreleptin antibodies with neutralizing activity in patients who develop severe infections or show signs suspicious for loss of MYALEPT efficacy during treatment. Contact Aegerion Pharmaceuticals, Inc. at 1-866-216-1526 for neutralizing antibody testing of clinical samples [see Adverse Reactions (6.2)].
5.2 Lymphoma
Three cases of T-cell lymphoma have been reported in the MYALEPT lipodystrophy program; all three patients had acquired generalized lipodystrophy. Two of these patients were diagnosed with peripheral T-cell lymphoma while receiving MYALEPT. Both had immunodeficiency and significant hematologic abnormalities including severe bone marrow abnormalities before the start of MYALEPT treatment. A separate case of anaplastic large cell lymphoma was reported in a patient receiving MYALEPT who did not have hematological abnormalities before treatment.
Lymphoproliferative disorders, including lymphomas, have been reported in patients with acquired generalized lipodystrophy not treated with MYALEPT. A causal relationship between MYALEPT treatment and the development and/or progression of lymphoma has not been established. Acquired lipodystrophies are associated with autoimmune disorders, and autoimmune disorders are associated with an increased risk of malignancies including lymphomas.
The benefits and risks of MYALEPT treatment should be carefully considered in patients with acquired generalized lipodystrophy and/or those with significant hematologic abnormalities (including leukopenia, neutropenia, bone marrow abnormalities, lymphoma, and/or lymphadenopathy).
5.3 MYALEPT REMS Program
MYALEPT is available only through a restricted distribution program under a REMS, called the MYALEPT REMS Program, because of the risks associated with the development of anti-metreleptin antibodies that neutralize endogenous leptin and/or MYALEPT and the risk for lymphoma [see Warnings and Precautions (5.1, 5.2)].
Notable requirements of the MYALEPT REMS Program include the following:
- Prescribers must be certified with the program by enrolling and completing training.
- Pharmacies must be certified with the program and only dispense MYALEPT after receipt of the MYALEPT REMS Prescription Authorization Form for each new prescription.
Further information is available at www.myaleptrems.com or 1-855-6MYALEPT.
5.4 Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
Dosage adjustments, including possible large reductions, of insulin or insulin secretagogue (e.g., sulfonylurea) may be necessary in some patients to minimize the risk of hypoglycemia [see Dosage and Administration (2.4) and Adverse Reactions (6.1)]. Closely monitor blood glucose in patients on concomitant insulin therapy, especially those on high doses, or insulin secretagogue (e.g., sulfonylurea), when treating with MYALEPT.
5.5 Autoimmunity
Leptin plays a role in immune system homeostasis. Acquired lipodystrophies are associated with autoimmune disorders including autoimmune hepatitis and membranoproliferative glomerulonephritis. Cases of progression of autoimmune hepatitis and membranoproliferative glomerulonephritis (associated with massive proteinuria and renal failure) were observed in some patients with acquired generalized lipodystrophy treated with MYALEPT. A causal relationship between MYALEPT treatment and the development and/or progression of autoimmune disease has not been established. The potential benefits and risks of MYALEPT treatment should be carefully considered in patients with autoimmune disease.
5.6 Hypersensitivity
There have been reports of generalized hypersensitivity (e.g., anaphylaxis, urticaria or generalized rash) in patients taking MYALEPT. If a hypersensitivity reaction occurs, instruct the patient to promptly seek medical advice regarding discontinuation of MYALEPT.
5.7 Benzyl Alcohol Toxicity
MYALEPT contains benzyl alcohol when reconstituted with BWFI. MYALEPT contains no preservative when reconstituted with sterile Water for Injection (WFI). Preservative-free WFI is recommended for use in neonates and infants. The preservative benzyl alcohol has been associated with serious adverse events and death in pediatric patients, particularly in neonates and premature infants [see Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Open-Label, Single-Arm Study
The safety of MYALEPT was evaluated in 48 patients with generalized lipodystrophy in a single-arm, open-label study [see Clinical Studies (14.1)]. The median duration of exposure in this trial was 2.7 years with a range of 3.6 months to 10.9 years. The most frequent adverse reactions are summarized in Table 3.
Table 3: Adverse Reactions of 5% or Greater Incidence in Patients with Generalized Lipodystrophy Receiving MYALEPT in an Open-Label, Single-Arm Study All Subjects
N=48 (%)1. Hypoglycemic events were assessed as mild, moderate, severe, or life threatening based on the protocol specified definitions: Mild: Documentation of low plasma glucose values with no symptoms; Moderate: Presence of clinical symptoms requiring ingestion of glucose, self-alleviated; Severe: Presence of neuroglycopenic symptoms requiring assistance from others for alleviation; Life threatening: Loss of consciousness and/or requiring intervention by administration of intravenous glucose or intramuscular glucagon.
Headache
6 (13)
Hypoglycemia1
6 (13)
Decreased weight
6 (13)
Abdominal pain
5 (10)
Arthralgia
4 (8)
Dizziness
4 (8)
Ear infection
4 (8)
Fatigue
4 (8)
Nausea
4 (8)
Ovarian cyst
4 (8)
Upper respiratory tract infection
4 (8)
Anemia
3 (6)
Back pain
3 (6)
Diarrhea
3 (6)
Paresthesia
3 (6)
Proteinuria
3 (6)
Pyrexia
3 (6)
In patients with generalized lipodystrophy receiving MYALEPT in this study, less common adverse reactions included injection-site erythema and urticaria (N=2 [4%]).
Six patients (13%) had 7 adverse reactions of hypoglycemia, 6 of which occurred in the setting of concomitant insulin use, with or without oral antihyperglycemic agents.
Two patients (4%) had events of pancreatitis, both of whom had a medical history of pancreatitis.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. Anti-metreleptin antibodies were detected in 84% (36/43) of generalized lipodystrophy patients studied in the MYALEPT trials. Total anti-metreleptin antibody titers ranged between 1:5 and 1:1,953,125. The incompleteness of the current immunogenicity database precludes understanding of the magnitude and persistence of the observed anti-drug antibody responses. Anti-metreleptin antibodies with neutralizing activity associated with adverse events consistent with loss of endogenous leptin activity and/or loss of MYALEPT efficacy were observed in 6% (2/33) of the patients with generalized lipodystrophy tested. Adverse events reported in these two patients included severe infections and worsening of metabolic control (increases in HbA1c and/or triglycerides). Test for anti-metreleptin antibodies with neutralizing activity in patients who develop severe infections or show signs suspicious for loss of MYALEPT efficacy during treatment. Contact Aegerion Pharmaceuticals, Inc. at 1-866-216-1526 for testing of clinical samples.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. The immunogenicity assays utilized in clinical trials lacked sensitivity, resulting in potential underestimation of the number of samples positive for anti-metreleptin antibodies with neutralizing activity. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to metreleptin with the incidence of antibodies to other products may be misleading.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of MYALEPT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Incorrect dose administered, accidental overdose [see Dosage and Administration (2.1, 2.3)]
-
7 DRUG INTERACTIONS
No formal drug interaction studies were performed.
Leptin is a cytokine and may have the potential to alter the formation of cytochrome P450 (CYP450) enzymes. This should be taken into account when prescribing concomitant drugs metabolized by CYP450 (e.g., oral contraceptives and drugs with a narrow therapeutic index). The effect of metreleptin on CYP450 enzymes may be clinically relevant for CYP450 substrates with narrow therapeutic index, where the dose is individually adjusted. Upon initiation or discontinuation of MYALEPT, in patients being treated with these types of agents, therapeutic monitoring of effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) should be performed and the individual dose of the agent adjusted as needed.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There is a program that monitors outcomes in women exposed to MYALEPT during pregnancy. Women who become pregnant during MYALEPT treatment are encouraged to enroll. Patients or their physicians should call 1-855-6MYALEPT to enroll.
Risk Summary
There are no adequate and well-controlled studies of MYALEPT in pregnant women. All pregnancies, regardless of drug exposure, have a background rate of 2% to 4% for major malformations and 15% to 20% for pregnancy loss. In a pre- and postnatal development study in mice, administration of metreleptin caused prolonged gestation and dystocia resulting in maternal death during parturition and lower survival of offspring in the immediate postnatal period at doses starting approximately at the maximum recommended clinical dose. Because animal reproduction studies are not always predictive of human response, MYALEPT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Clinical Considerations
Disease-Associated Maternal and Fetal Risk
The contribution of MYALEPT to obstetrical risks and complications is unknown compared with those already documented in the lipodystrophy patient population (e.g., gestational diabetes, macrosomia, eclampsia, intrauterine growth retardation, intrauterine death, and miscarriage).
Labor and Delivery
The effects of MYALEPT on labor and delivery in pregnant women are unknown. In an in vitro study of human myometrial tissue exposed to a recombinant leptin, human uterine contractility was inhibited. Furthermore, prolonged gestation and dystocia were observed in animal studies with metreleptin (see below).
Animal Data
Metreleptin administered to pregnant mice during the period of organogenesis was not teratogenic at doses ranging between 7- and 15-fold the maximum recommended clinical dose, based on body surface area of a 20- and 60-kg patient, respectively.
In a pre- and postnatal development study in mice, metreleptin administered at doses of 3, 10, and 30 mg/kg (approximately 1-, 5-, and 15-fold the clinical dose for a 60-kg subject, based on body surface area) from gestation day 6 to lactation day 21 caused prolonged gestation and dystocia at all doses, starting at approximately the maximum recommended clinical dose. Prolonged gestation resulted in the death of some females during parturition and lower survival of offspring within the immediate postnatal period. Consistent with metreleptin pharmacology, decreased maternal body weight was observed from gestation throughout lactation at all doses and resulted in reduced weight of offspring at birth, which persisted into adulthood. However, no developmental abnormalities were observed and reproductive performance of the first or second generations was not affected at any dose.
Placental transfer of metreleptin into the fetus was low (approximately 1%) following subcutaneous dosing.
8.3 Nursing Mothers
It is not known if MYALEPT is present in human milk. Endogenous leptin is present in human milk. Because of the potential for serious adverse reactions (including possible adverse reactions related to passage of anti-metreleptin antibodies) in nursing infants from MYALEPT a decision should be made whether to discontinue nursing or discontinue the drug, taking into account importance of drug to the mother [see Adverse Reactions (6.2) and Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The MYALEPT study included a total of 35 pediatric patients (73%) with an age range from 1 to 17 years [see Clinical Studies (14.1)]. No clinically meaningful differences were observed in the efficacy and safety of MYALEPT between pediatric and adult patients.
MYALEPT contains benzyl alcohol when reconstituted with BWFI. MYALEPT contains no preservative when reconstituted with WFI. Preservative-free WFI is recommended for use in neonates and infants. The preservative benzyl alcohol has been associated with serious adverse events and death, particularly in pediatric patients. The "gasping syndrome" (characterized by central nervous system depression, metabolic acidosis, gasping respirations, and high levels of benzyl alcohol and its metabolites found in the blood and urine) has been associated with benzyl alcohol dosages >99 mg/kg/day in neonates and low-birth weight infants. Additional symptoms may include gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse.
Although normal therapeutic doses of this product deliver amounts of benzyl alcohol that are substantially lower than those reported in association with the "gasping syndrome," the minimum amount of benzyl alcohol at which toxicity may occur is not known. Premature and low-birth-weight infants, as well as patients receiving high dosages, may be more likely to develop toxicity. Practitioners administering this and other medications containing benzyl alcohol should consider the combined daily metabolic load of benzyl alcohol from all sources. When reconstituted with 2.2 mL of BWFI, MYALEPT contains 1.76 mg of benzyl alcohol per mg of metreleptin or 9 mg of benzyl alcohol per mL of reconstituted product.
8.5 Geriatric Use
Clinical trials of MYALEPT did not include sufficient numbers of subjects aged 65 and over (n=1) to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
In one post-marketing case, a dose miscalculation resulted in an infant being exposed to a 10-fold overdose of metreleptin for 8 months. In this case, prolonged overdose was associated with severe anorexia causing vitamin and zinc deficiencies, iron deficiency anemia, protein calorie malnutrition, and poor weight gain, which resolved following supportive treatment and dose adjustment.
In the event of an overdose, patients should be monitored and appropriate supportive treatment be initiated as dictated by the patient's clinical status.
-
11 DESCRIPTION
MYALEPT (metreleptin) for injection is a recombinant human leptin analog for injection that binds to and activates the leptin receptor. Metreleptin (recombinant methionyl-human leptin) is produced in E. coli and differs from native human leptin by the addition of a methionine residue at its amino terminus. Metreleptin is a 147-amino acid, nonglycosylated, polypeptide with one disulfide bond between Cys-97 and Cys-147 and a molecular weight of approximately 16.15 kDa.
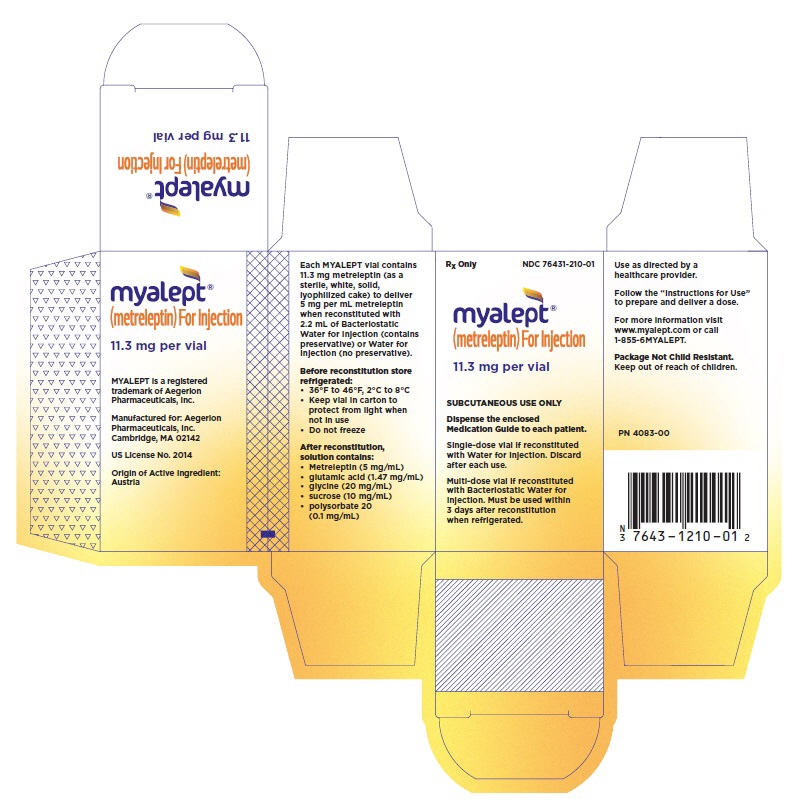
MYALEPT is supplied as a sterile, white, solid, lyophilized cake containing 11.3 mg that is reconstituted with 2.2 mL of BWFI or WFI to a final formulation of 5 mg/mL metreleptin for subcutaneous injection. Inactive ingredients are: glutamic acid (1.47 mg/mL), glycine (20 mg/mL), polysorbate 20 (0.1 mg/mL), and sucrose (10 mg/mL), pH 4.25.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Adipocytes store lipids to meet the fuel requirements of non-adipose tissues during fasting. In patients with generalized lipodystrophy, the deficiency of adipose tissue leads to hypertriglyceridemia and ectopic deposition of fat in non-adipose tissues such as liver and muscle, contributing to metabolic abnormalities including insulin resistance. Native leptin is a hormone predominantly secreted by adipose tissue that informs the central nervous system of the status of energy stores in the body. In patients with generalized lipodystrophy, leptin deficiency, resulting from the loss of adipose tissue, contributes to excess caloric intake, which exacerbates the metabolic abnormalities.
MYALEPT (metreleptin) for injection exerts its function by binding to and activating the human leptin receptor (ObR), which belongs to the Class I cytokine family of receptors that signals through the JAK/STAT transduction pathway.
12.2 Pharmacodynamics
Clinical studies in patients with generalized lipodystrophy suggest that MYALEPT increases insulin sensitivity and reduces food intake. Improvements in insulin sensitivity and reductions in food intake are consistent with lower HbA1c, fasting glucose, and fasting triglyceride values that were seen in the MYALEPT clinical trial [see Clinical Studies (14)].
12.3 Pharmacokinetics
There are limited data on the pharmacokinetics of metreleptin in patients with generalized lipodystrophy, and therefore, no formal exposure-response analysis has been performed. It should be noted that the leptin assay measures both endogenous leptin as well as exogenously administered metreleptin.
Absorption
Peak serum leptin concentration (Cmax) occurred approximately 4.0 to 4.3 hours after subcutaneous administration of single doses ranging from 0.1 to 0.3 mg/kg in healthy subjects. In a supportive trial in lipodystrophy patients, the median Tmax of metreleptin was 4 hours (range: 2 to 8 hours; N=5) following single-dose administration of metreleptin.
Distribution
In studies of healthy adult subjects, following intravenous administration of metreleptin, leptin volume of distribution was approximately 4 to 5 times plasma volume; volumes (Vz) (mean ± SD) were 370 ± 184 mL/kg, 398 ± 92 mL/kg, and 463 ± 116 mL/kg for 0.3, 1.0, and 3.0 mg/kg/day doses, respectively.
Metabolism and Elimination
No formal metabolism studies have been conducted with metreleptin. Nonclinical data indicate renal clearance is the major route of metreleptin elimination, with no apparent contribution of systemic metabolism or degradation. Following single subcutaneous doses of 0.01 to 0.3 mg/mL metreleptin in healthy subjects, the half-life was 3.8 to 4.7 hours. The clearance of metreleptin is expected to be delayed in the presence of leptin antibodies [see Adverse Reactions (6.2)].
Drug Interactions
No drug interaction studies have been conducted in lipodystrophy patients [see Drug Interactions (7)].
Specific Populations
Renal Impairment
No formal pharmacokinetic studies were conducted in patients with renal impairment. Nonclinical data indicate renal clearance is the major route of metreleptin elimination, with no apparent contribution of systemic metabolism or degradation. Hence, the pharmacokinetics of metreleptin may be altered in subjects with renal impairment.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies in rodents have not been conducted with metreleptin. No proliferative or preneoplastic lesions were observed in mice or dogs following treatment up to six months. However, leptin is reported in the literature to promote cell proliferation in vitro and tumor progression in some mouse models of cancer.
Metreleptin was not mutagenic in the Ames bacterial mutagenicity assay or clastogenic in an in vitro chromosomal aberration assay in Chinese hamster ovary cells and human peripheral blood lymphocytes. Metreleptin was not mutagenic or clastogenic in an in vivo mouse micronucleus assay.
In a fertility study in mice, metreleptin had no adverse effects on mating, fertility, or early embryonic development at doses ranging between 7 and 15 times the maximum recommended clinical dose based on body surface area of a 20- and 60-kg patient, respectively.
-
14 CLINICAL STUDIES
14.1 Open-Label, Single-Arm Study
An open-label, single-arm study evaluated MYALEPT treatment in patients with congenital or acquired generalized lipodystrophy and diabetes mellitus, hypertriglyceridemia, and/or increased fasting insulin.
Baseline Disease Characteristics and Demographics
Of the 48 patients enrolled, 32 (67%) had congenital generalized lipodystrophy and 16 (33%) had acquired generalized lipodystrophy. Overall, 36 (75%) patients were female, 22 (46%) were Caucasian, 10 (21%) Hispanic, and 9 (19%) Black. The median age at baseline was 15 years (range: 1 - 68 years), with 35 (73%) patients being less than 18 years of age. The median fasting leptin concentration at baseline was 0.7 ng/mL in males (range: 0.3 - 3.3 ng/mL) and 1.0 ng/mL in females (range: 0.3 - 3.3 ng/mL).
Treatment Duration and Dosage in the Study
The median duration of MYALEPT treatment was 2.7 years (range: 3.6 months - 10.9 years). MYALEPT was administered subcutaneously either once daily or twice daily (in two equal doses). The weighted average daily dose (i.e., the average dose taking into account duration of treatment at different doses) for the 36 patients with baseline body weight greater than 40 kg was 2.6 mg for males and 4.6 mg for females during the first year of treatment, and 3.2 mg for males and 6.3 mg for females over the entire study period. For the 12 patients with baseline body weight less than 40 kg, the weighted average daily dose was 0.06 to 0.11 mg/kg (0.8-4.3 mg) over the entire study period.
Efficacy Results
At baseline, 37 (77%) patients had HbA1c values of 7% or greater, 19 (40%) had HbA1c values of 9% or greater, 33 (69%) had fasting plasma glucose values of 126 mg/dL or greater, 17 (35%) had fasting triglyceride values of 500 mg/dL or greater, and 11 (23%) had fasting triglyceride values of 1000 mg/dL or greater.
Patients treated with MYALEPT had mean/median reductions in HbA1c, fasting glucose, and triglycerides at 1 year (Table 4). The changes in HbA1c, fasting glucose, and triglycerides observed at Month 4 were similar to those at 1 year. Concomitant antihyperglycemic and lipid-altering medication dosage regimens were not held constant during the study; for example, some patients treated with insulin had their dosage increased and others had large reductions or discontinuation of insulin.
Table 4: Results in an Open-Label, Single-Arm Study in Patients with Generalized Lipodystrophy Treated with MYALEPT (N=48) Parameter n Baseline Change from Baseline at Month 12 SD = standard deviation; Q = quartile Mean (SD)
Mean (SD)
HbA1c (%)
35
8.5 (2)
−2 (1.5)
Fasting Glucose (mg/dL)
37
174 (85)
−49 (75)
Median (Q1, Q3)
Median Change (Q1, Q3)
Fasting Triglycerides (mg/dL)
36
348 (176, 769)
−184 (−643, −21)
Median Percent Change (Q1, Q3)
−55 (−77, −20)%
Among 28 patients with generalized lipodystrophy who had a baseline HbA1c 7% or greater and data available at Month 12, the mean (SD) baseline HbA1c was 9.3 (1.5%) and the mean reduction in HbA1c at Month 12 was 2.4%.
Among 12 patients with generalized lipodystrophy who had a baseline triglyceride level 500 mg/dL or greater and data available at Month 12, the median baseline triglyceride level was 1527 mg/dL and the median reduction in triglycerides at Month 12 was 1117 mg/dL.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
- MYALEPT (metreleptin) for injection for subcutaneous administration is supplied in a single carton containing one vial for reconstitution (NDC: 76431-210-01).
- Each vial contains 11.3 mg metreleptin (as a sterile, white, solid, lyophilized cake) to deliver 5 mg per mL of metreleptin when reconstituted with 2.2 mL of BWFI or WFI.
16.2 Storage and Handling
- MYALEPT should be stored in the refrigerator at 36°F to 46°F (2°C to 8°C) and protected from light until preparing for use. Keep MYALEPT vials in the carton when not in use.
- MYALEPT should not be used past the expiration date.
- Do not freeze MYALEPT.
- Do not use if the white lyophilized cake is discolored.
- Use with BWFI: when MYALEPT is reconstituted with BWFI, the vial can be used for multiple doses within 3 days when stored in the refrigerator at 36°F to 46°F (2°C to 8°C) and protected from light.
- Use with WFI: when MYALEPT is reconstituted with WFI, the vial can be used for a single dose should be administered immediately. Unused reconstituted solution cannot be saved for later use and should be discarded.
- After reconstitution, the vials should not be frozen (below 0°C) or shaken vigorously. If the reconstituted product is inadvertently frozen, it should be thrown away.
- After reconstitution, the mixture should be clear and colorless. Do not use if visible particulates are present in the solution.
- Keep out of the reach of children.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved Patient Labeling (Medication Guide).
Risk of Neutralizing Antibodies
Advise patients that neutralizing antibodies may result in loss in activity of endogenous leptin or loss of efficacy of MYALEPT. Advise patients on symptoms or signs that would warrant antibody testing [see Warnings and Precautions (5.1) and Adverse Reactions (6.2)].
Risk of Lymphoma
Advise patients that lymphoma has been reported in patients both treated and not treated with MYALEPT. Advise patients on symptoms or signs that indicate changes in hematologic status and the importance of routine laboratory assessments and physician monitoring [see Warnings and Precautions (5.2)].
Risk of Hypoglycemia
Advise patients that the risk of hypoglycemia is increased when MYALEPT is used in combination with insulin or an insulin secretagogue (e.g., sulfonylurea). Explain the symptoms, treatment, and conditions that predispose to development of hypoglycemia to the patient. Advise patients who are taking concomitant insulin, especially those on high doses, or an insulin secretagogue, to closely monitor blood glucose. Hypoglycemia management should be reviewed and reinforced when initiating MYALEPT therapy, particularly when concomitantly administered with insulin or an insulin secretagogue [see Warnings and Precautions (5.4)].
Risk of Autoimmune Disease
Advise patients that worsening of autoimmune disease has been reported during the clinical study of MYALEPT. Advise patients with a history of autoimmune disease on symptoms or signs that indicate exacerbation of underlying autoimmune disease and the importance of routine laboratory assessments and physician monitoring [see Warnings and Precautions (5.5)].
Risk of Hypersensitivity Reactions
Inform patients that hypersensitivity reactions have been reported during use of MYALEPT. If symptoms of hypersensitivity reactions occur, patients should seek medical advice [see Warnings and Precautions (5.6)].
Nursing Mothers
Advise nursing mothers that breastfeeding is not recommended with MYALEPT use [see Use in Specific Populations (8.3)].
Instructions
- Inform patients that each vial of MYALEPT requires reconstitution with BWFI or preservative-free WFI, and administration as subcutaneous injection using a syringe and needle. Injections can be given at any time of the day, with or without meals.
- Patients and caregivers should receive proper training in how to prepare and administer the correct dose of MYALEPT prior to self-administration. The first dose of MYALEPT should be administered by the patient or caregiver under the supervision of a qualified healthcare professional.
- Advise patients on appropriate syringe for administration, dosing regimen, injection technique, and the importance of proper storage of MYALEPT. Care should be taken to avoid intramuscular injection, especially in patients with minimal subcutaneous adipose tissue.
- Advise patients to read the Instructions for Use for complete administration instructions. The MYALEPT Medication Guide and Instructions for Use should be reviewed before starting therapy and each time the prescription is refilled.
- When discontinuing MYALEPT in patients with a history of pancreatitis and/or severe hypertriglyceridemia, instruct patients to taper their dose over a one-week period. Advise patients that additional monitoring of triglyceride levels and possible initiation or dose adjustment of lipid-lowering medications may be considered [see Dosage and Administration (2.5)].
- SPL UNCLASSIFIED SECTION
-
Medication Guide
MYALEPT® (MAI-uh-lept)
(metreleptin) for injection
for subcutaneous useRead this Medication Guide that comes with MYALEPT before you start using it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about MYALEPT?
MYALEPT may cause serious side effects, including:
-
risk for loss of endogenous leptin activity or loss of MYALEPT efficacy due to neutralizing antibodies.
Some people who use MYALEPT make antibodies in their blood that may reduce how well the leptin in your body (endogenous) works or how well MYALEPT works. Side effects may include:- infection
- problems with blood sugar, including diabetes
- an increase in the amount of fat in your blood (triglycerides)
- lymphoma (a type of blood cancer). There may be an increased risk of getting lymphoma when you use MYALEPT.
MYALEPT is only available through a restricted program called the MYALEPT Risk Evaluation and Mitigation Strategy (REMS) Program. For more information about the MYALEPT REMS Program go to www.myaleptrems.com or call 1-855-6MYALEPT.
What is MYALEPT?
MYALEPT is a prescription medicine used with a diet recommended by your healthcare provider to treat problems caused by not having enough leptin in your body (leptin deficiency) in people with congenital or acquired generalized lipodystrophy.
- It is not known if MYALEPT is safe and effective when used:
- to treat problems (complications) caused by partial lipodystrophy
- to treat liver disease, including non-alcoholic steatohepatitis (NASH)
- MYALEPT should not be used to treat:
- people with HIV-related lipodystrophy
- people with metabolic disease, including diabetes mellitus and hypertriglyceridemia, without signs or symptoms of congenital or acquired generalized lipodystrophy
Who should not use MYALEPT?
Do not use MYALEPT if you:
- have general obesity that is not caused by a congenital leptin deficiency.
- are allergic to metreleptin or any of the ingredients in MYALEPT. See the end of this Medication Guide for a complete list of ingredients in MYALEPT.
Talk to your healthcare provider right away if you have any symptoms of an allergic reaction including a rash or itching (hives).
Symptoms of a severe allergic reaction may include:- swelling of your face, lips, tongue, or throat
- problems breathing or swallowing
- severe rash or itching
- fainting or feeling dizzy
- very rapid heartbeat
What should I tell my healthcare provider before using MYALEPT?
Before using MYALEPT, tell your healthcare provider if you:
- have or have had problems with your blood cells including low blood cell counts, especially white blood cells
- have or have had problems with your bone marrow
- have or have had swollen lymph nodes (lymphadenopathy)
- have or have had lymphoma
- use insulin or a sulfonylurea
- have or have had problems with your immune system (autoimmune disease)
- have or have had problems with your pancreas (pancreatitis)
- have high blood triglyceride levels
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if MYALEPT will harm your unborn baby
- If you become pregnant while using MYALEPT, talk to your healthcare provider about registering with a program to collect information about the outcomes of moms and babies exposed to MYALEPT during pregnancy. You can enroll in the MYALEPT program by calling 1-855-6MYALEPT.
- are breastfeeding or plan to breastfeed. It is not known if MYALEPT passes into your breast milk. You and your healthcare provider should decide if you will take MYALEPT or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist when you get a new medicine.
How should I use MYALEPT?
See the Instructions for Use that come with MYALEPT for detailed instructions on using MYALEPT the right way.
- Use MYALEPT exactly as your healthcare provider tells you to.
- Your healthcare provider will tell you how much MYALEPT to use and when to use it. Do not change your dose unless your healthcare provider tells you to.
- Do not suddenly stop using MYALEPT. Stopping MYALEPT suddenly may cause a serious problem with your pancreas (pancreatitis) and very high triglycerides.
- If your healthcare provider decides that you should stop using MYALEPT, your healthcare provider should slowly decrease your dose (taper) over 1 week.
- MYALEPT is injected 1 time a day at the same time each day.
- MYALEPT can be used with or without food.
- If you miss a dose of MYALEPT, take it as soon as you remember. Take your regular dose the next day at your normal time. Do not take more than your regular daily dose in a single day. Do not take an extra dose or increase the amount of your dose to make up for a missed dose.
- Do not mix MYALEPT and insulin in the same syringe or vial. Although MYALEPT and insulin doses may be given at the same time, do not inject MYALEPT and insulin in the same injection site.
- When MYALEPT is used in newborns or infants, your healthcare provider will tell you if MYALEPT should be mixed with a liquid called sterile water for injection (WFI) (preservative-free). For older children and adults, bacteriostatic water for injection (BWFI) may be used.
- MYALEPT is given as an injection under the skin (subcutaneous) of your stomach (abdomen), thigh, or upper arm. Do not inject MYALEPT into a vein or muscle.
What are the possible side effects of MYALEPT?
MYALEPT can cause serious side effects, including:
- See "What is the most important information I should know about MYALEPT?"
-
low blood sugar (hypoglycemia). You may get low blood sugar if you use MYALEPT with another medicine that can cause low blood sugar, such as insulin or sulfonylurea. The dose of your insulin or sulfonylurea may need to be lowered while you use MYALEPT. Signs and symptoms of low blood sugar may include:
- shakiness
- sweating
- headache
- drowsiness
- weakness
- dizziness
- confusion
- irritability
- hunger
- fast heart beat
- feeling jittery
Talk with your healthcare provider about how to recognize and treat low blood sugar. Make sure that your family and other people around you a lot know how to recognize and treat low blood sugar.
- autoimmunity. People who have or have had certain problems with their immune system (autoimmune disease) may have worsening of their symptoms with MYALEPT. Talk to your healthcare provider about what symptoms you should watch for that would warrant further testing.
- allergic reactions (hypersensitivity). Allergic reactions can happen in people who use MYALEPT. Talk to your healthcare provider right away if you have any symptom of an allergic reaction. See "Who should not take MYALEPT?"
- benzyl alcohol toxicity. Serious side effects including death have happened in newborns or infants who have received the preservative benzyl alcohol. MYALEPT, when mixed with a liquid called bacteriostatic water for injection (BWFI), contains benzyl alcohol. MYALEPT, when mixed with a liquid called sterile water for injection (WFI) (preservative-free), contains no benzyl alcohol. When MYALEPT is used in newborns or infants, MYALEPT should be mixed with sterile water for injection (WFI).
The most common side effects of MYALEPT include:
- headache
- low blood sugar (hypoglycemia)
- decreased weight
- abdominal pain
Talk to your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the side effects with MYALEPT. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store MYALEPT?
- Store MYALEPT in the refrigerator between 36°F to 46°F (2°C to 8°C).
- KEEP MYALEPT vials in their carton and out of the light.
- Do not freeze MYALEPT.
- Do not use MYALEPT past the expiration date printed on the vial.
- Do not use MYALEPT if the white powder in the vial is discolored.
- After mixing, the MYALEPT liquid in the vial should be clear and colorless. Do not use MYALEPT if it is colored or cloudy, or has any lumps or particles in it. Throw the vial away and get a new one.
- After mixing, do not freeze or shake MYALEPT.
-
MYALEPT mixed with BWFI:
- MYALEPT can be used for more than 1 dose for up to 3 days when stored in the refrigerator between 36°F to 46°F (2°C to 8°C) and out of the light. Throw away any unused MYALEPT after 3 days.
-
MYALEPT mixed with WFI:
- Should be used right away. Throw away any unused MYALEPT, it cannot be saved for later use.
Keep MYALEPT and all medicines out of the reach of children.
General information about the safe and effective use of MYALEPT
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use MYALEPT for a condition for which it was not prescribed. Do not give MYALEPT to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about MYALEPT. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about MYALEPT that is written for health professionals.
For more information about MYALEPT, go to www.myalept.com or call MYALEPT Customer Service at 1-855-6MYALEPT.
What are the ingredients in MYALEPT?
Active Ingredient: metreleptin
Inactive Ingredients: glutamic acid, glycine, sucrose, and polysorbate 20
This Medication Guide has been approved by the U.S. Food and Drug Administration.
MYALEPT is a registered trademark of Aegerion Pharmaceuticals, Inc.
Manufactured for:
Aegerion Pharmaceuticals, Inc.
Cambridge, MA 02142
PN 4087-00
Approved: August 2015
-
risk for loss of endogenous leptin activity or loss of MYALEPT efficacy due to neutralizing antibodies.
-
INSTRUCTIONS FOR USE
Instructions for Use
MYALEPT® (MAI-uh-lept)
(metreleptin) for injection
Vial
- A healthcare provider should show you how to inject MYALEPT before you use it for the first time. A healthcare provider should also watch you inject your MYALEPT dose the first time you inject it.
- Do not inject MYALEPT until your healthcare provider has shown you the right way to inject it. If you have questions or do not understand the instructions, talk to your healthcare provider or pharmacist.
- MYALEPT is only for use under the skin (subcutaneous).
- Do not share your MYALEPT needles with another person. You may give an infection to them, or get an infection from them.
This Instructions for Use is divided into 6 steps:
Step 1: Getting started
Step 2: Filling the 3 mL syringe with 2.2 mL of liquid
Step 3: Preparing MYALEPT
Step 4: Filling the syringe used for injecting MYALEPT
Step 5: Injecting MYALEPT
Step 6: Disposing of used needles and syringes
If at any time during these steps you have questions:
- See the "Common Questions" tab
- Call 1-855-6MYALEPT
- Visitwww.MYALEPT.com
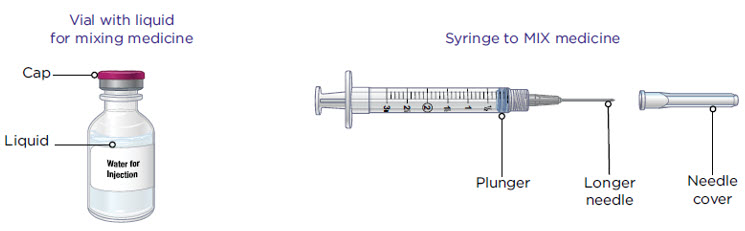
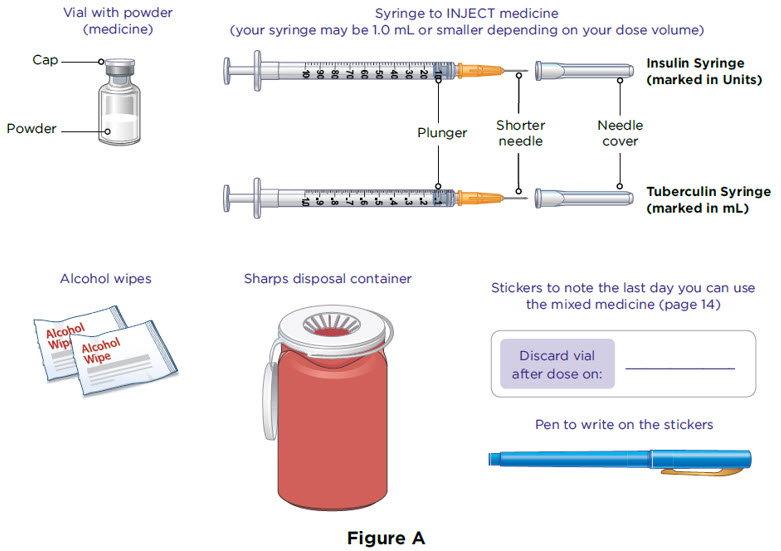
Supplies you will need to give your MYALEPT (see Figure A).
Make sure you have all the supplies listed below BEFORE using MYALEPT.
You can get these supplies with a prescription from your healthcare provider, from a retail or hospital pharmacy, or the specialty pharmacy that distributes MYALEPT.
- a vial with liquid for mixing MYALEPT
- Sterile water for injection should be used in newborns and infants
- Bacteriostatic water for injection should be used for older children and adults
- a 3 mL syringe with a longer needle for mixing MYALEPT
- a vial with MYALEPT powder
- a 1 mL syringe (or smaller) with a shorter needle for injecting MYALEPT
- 2 alcohol wipes
- 1 sharps container for throwing away used needles and syringes. See "Disposing of used needles and syringes" at the end of these instructions.
- Stickers to note discard date for mixed medicine
How to read your syringes:
The 3 mL syringe has a longer needle (see Figure B).
The 3 mL syringe is the syringe you will use to mix MYALEPT. Always fill the 3 mL syringe with 2.2 mL of liquid. Do not inject yourself with the 3 mL syringe.
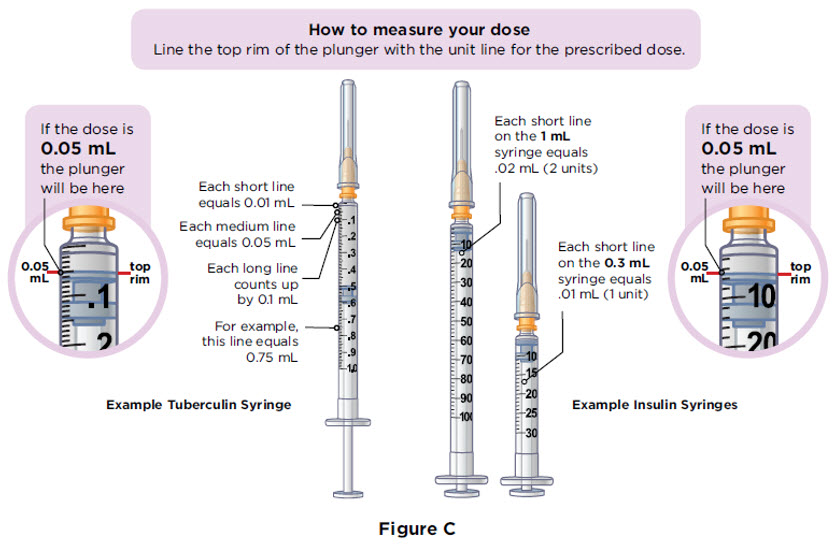
The injection syringes have a shorter needle (see Figure C).
- People who weigh more than 88 pounds (lbs) (40 kg) should use a 1 mL syringe to administer their injection of MYALEPT. People who weigh 88 lbs (40 kg) or less should use either a 1 mL or smaller syringe because smaller amounts of MYALEPT are needed for injection. Using the wrong size syringe can cause you to measure the wrong amount of MYALEPT.
The 1 mL (or smaller) syringe is the syringe you will use to inject MYALEPT. Only use the 1 mL (or smaller) syringe to inject your dose of MYALEPT.
Step 1: Getting Started
Your dose of MYALEPT may change over time, depending on how MYALEPT works for you. So it is important to keep track of your dose. On the line below, write down your dose in mL and the date. Be sure to keep this up-to-date if your dose changes:
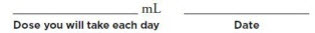
- Take 1 MYALEPT vial out of the refrigerator 10 minutes before you plan to inject to allow it to reach room temperature.
- Set 1 vial with the liquid you will need to mix MYALEPT on your work surface.
- Check the powder in the MYALEPT vial. It should be white. Do not use MYALEPT if the powder is discolored. Throw it away and get a new one.
- Check the expiration date printed on the MYALEPT vial. Do not use MYALEPT past the expiration date printed on the vial (see Figure D).
Figure D
For this step, you will need:
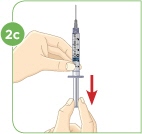
STEP 2: Filling the 3 mL syringe (used to mix MYALEPT) with 2.2 mL of liquid
For this step, you will need:
A 3 mL syringe (with longer needle) used to mix MYALEPT

Vial with liquid
(from Step 1)
Sharps disposal container

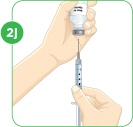
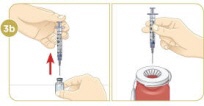
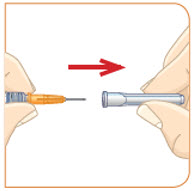
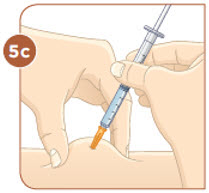
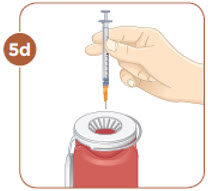
Set the vial with the liquid on the work surface. Insert the needle into the top of the vial.
Push the plunger down all the way to fill the vial with air.
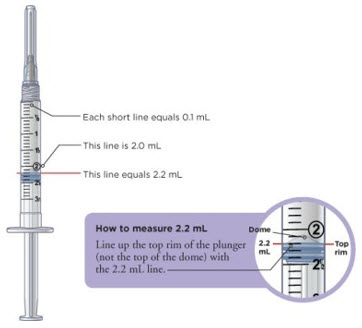
Tip: You will always fill the 3 mL syringe with 2.2 mL of liquid.
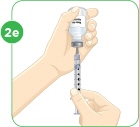
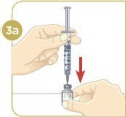
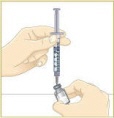
STEP 3: Preparing MYALEPTNote: If you already mixed your MYALEPT before today, go to "Using a vial of mixed MYALEPT" at the end of this step.
Option 1: Mixing a new vial of MYALEPT:
For this step, you will need:
The 3 mL syringe filled with 2.2 mL of liquid (from Step 2)

Vial with powder (from Step 1)

Sharps disposal container

Tip: If your vial of MYALEPT is not mixed well, go back to Step 3c.
Note: Go to Step 4 if you just mixed a new vial of MYALEPT.Option 2: Using a vial of mixed MYALEPT:
Note: For newborns or infants using MYALEPT, throw away any unused mixed MYALEPT right away. Do not store it for reuse.
For this step, you will need:
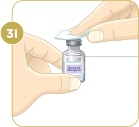
Choose a clean, flat work surface large enough to let you prepare the medicine.
Important: Do not mix any liquid or mixed medicine from another vial with the vial you just cleaned.
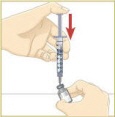
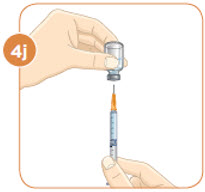
STEP 4: Filling the syringe used for injecting MYALEPT
Patients greater than 40 kg should use a 1 mL syringe for administration. In pediatric patients, small volumes for administration can result in medication errors when measured incorrectly. Smaller syringe sizes may be more appropriate for pediatric patients weighing less than or equal to 25 kg. Ensure the proper size syringe is selected.
The table below shows how a dose of MYALEPT (mg) is converted to solution volume (mL) and the injection syringe marks (units) that correspond with the dose.
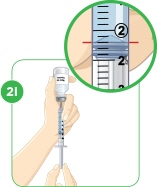
Conversion of MYALEPT Dose to Unit Measurement for Injection DOSE OF MYALEPT AMOUNT OF MIXED MYALEPT SOLUTION TO INJECT (TUBERCULIN SYRINGE USERS) AMOUNT OF MIXED MYALEPT SOLUTION TO INJECT (INSULIN SYRINGE USERS) 0.30 mg 0.06 mL 6 units 0.36 mg 0.07 mL 7 units 0.42 mg 0.08 mL 8 units 0.48 mg 0.09 mL 9 units 0.54 mg 0.10 mL 10 units 0.60 mg 0.12 mL 12 units 0.66 mg 0.13 mL 13 units 0.72 mg 0.14 mL 14 units 0.78 mg 0.15 mL 15 units 0.84 mg 0.16 mL 16 units 0.90 mg 0.18 mL 18 units 0.96 mg 0.19 mL 19 units 1.02 mg 0.20 mL 20 units 1.08 mg 0.21 mL 21 units 1.14 mg 0.22 mL 22 units 1.20 mg 0.24 mL 24 units 1.26 mg 0.25 mL 25 units 1.32 mg 0.26 mL 26 units 1.38 mg 0.27 mL 27 units 1.44 mg 0.28 mL 28 units 1.50 mg 0.30 mL 30 units 1.56 mg 0.31mL 31 units 1.62 mg 0.32 mL 32 units 1.68 mg 0.33 mL 33 units 1.74 mg 0.34 mL 34 units 1.80 mg 0.36 mL 36 units 1.86 mg 0.37 mL 37 units 1.92 mg 0.38 mL 38 units 1.98 mg 0.39 mL 39 units 2.04 mg 0.40 mL 40 units 2.10 mg 0.42 mL 42 units 2.16 mg 0.43 mL 43 units 2.22 mg 0.44 mL 44 units 2.28 mg 0.45 mL 45 units 2.34 mg 0.46 mL 46 units 2.40 mg 0.48 mL 48 units 2.5 mg 0.50 mL 50 units 3.75 mg 0.75 mL 75 units 5.00 mg 1.00 mL 100 units 6.25 mg 1.25 mL 125 units 7.50 mg 1.5 mL 150 units 8.75 mg 1.75 mL 175 units 10.00 mg 2.00 mL 200 units For this step, you will need:
A (1 mL or smaller) syringe (with shorter needle) used to inject MYALEPT
Examples: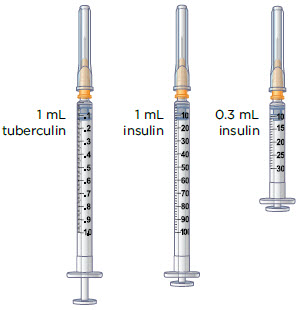
Vial of mixed MYALEPT (from Step 3)

Sharps disposal container

 Remove the injection syringe from the plastic wrapper. Always use a new syringe.
Remove the injection syringe from the plastic wrapper. Always use a new syringe. 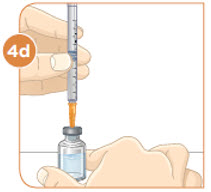
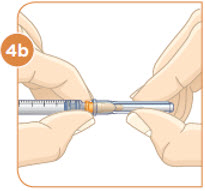
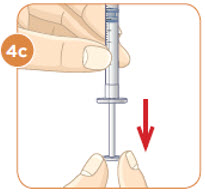
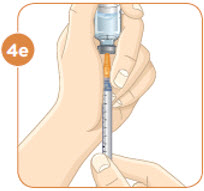
Hold the vial with the mixed MYALEPT. Insert the needle into the top of the MYALEPT vial.
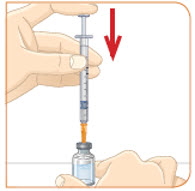
Push the plunger down all the way, to fill the vial with air.
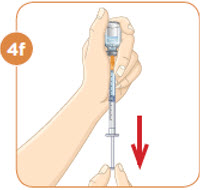
Tip: If your prescribed dose is more than 1 mL, you will need to use 2 separate injections to take your full daily dose. Repeat Step 4 to fill the second syringe.
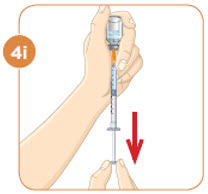
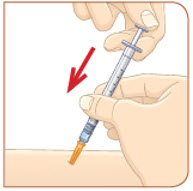
STEP 5: Injecting MYALEPT
For this step, you will need:
Syringe filled with your
MYALEPT dose
(from Step 4)
Alcohol wipe
(from Step 1)
Sharps disposal
container for used syringes and needles
Important:
Inject MYALEPT under the skin (subcutaneous). Do not inject MYALEPT into a muscle or vein.
Important:
Unused MYALEPT mixed with sterile water for injection (newborns or infants), must be thrown away.
Do not store it for reuse.
MYALEPT mixed with bacteriostatic water for injection can be stored for reuse.
Important:
Stickers to Note the Last Day You Can Use MYALEPT When Mixed with Bacteriostatic Water for Injection
STEP 6: Disposing of used needles and syringes:
- Put your used needles and syringes in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
Common questions
Questions about storing and traveling with MYALEPT
page 15
Questions about preparing and mixing your dose of MYALEPT
pages 15-16
Questions about injecting MYALEPT
page 16
Questions about MYALEPT supplies
page 16
Common questions
Questions about storing and traveling with MYALEPT
Questions about preparing and mixing your dose of MYALEPT
Questions about injecting MYALEPT
13Q
What are some tips for injecting MYALEPT?
A
Try these tips to help you prepare for your injection with MYALEPT:
- Know the steps in the process. To get to know the steps, read this booklet ahead of time. It may help to read the steps out loud. If you have questions about how to inject, make sure to ask your healthcare provider
- Find a place that is clean and well lit in which you can do the injection
- Ask for help. You may feel more comfortable if a care partner does the injection for you
- Put ice on the injection site. You may want to use ice at the injection site, after your injection, to help lessen some of the pain you might feel
- Make sure the vial with powder (MYALEPT) is at room temperature before you mix and inject it. To do this, take a vial with powder out of the refrigerator and set it out for 10 to 15 minutes
Questions about MYALEPT supplies
14Q
How do I obtain the supplies needed to inject MYALEPT?
A
The standard supplies needed to inject MYALEPT include needles, syringes, liquid for mixing and a disposal container. Your healthcare provider will give you a prescription for these supplies which can be filled by a retail or hospital pharmacy, or the specialty pharmacy who distributes MYALEPT.
15Q
How do I store the other supplies that are needed to prepare and inject a dose of MYALEPT?
A
Store syringes, vials of liquid with colored caps, alcohol wipes, and sharps disposal container at room temperature or at the temperature that is written on the package they came in. Be sure to check the expiration date on the packages of your supplies before use. Do not use expired supplies.
To learn more about MYALEPT
- Talk with your healthcare provider
- Read the Medication Guide that came with MYALEPT. The Medication Guide can help answer your questions about MYALEPT, such as what it is used for, possible side effects, and when to take it
- Visit www.MYALEPT.com or call 1-855-6MYALEPT for FREE ongoing support and services
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
MYALEPT is a registered trademark of Aegerion Pharmaceuticals, Inc.
Manufactured for:
Aegerion Pharmaceuticals, Inc.
Cambridge, MA 02142
PN 4086-01
Issued December 2019
- PRINCIPAL DISPLAY PANEL - NDC: 76431-210-01 - 11.3 mg Carton
- PRINCIPAL DISPLAY PANEL - NDC: 76431-210-01 - 11.3 mg Vial
-
INGREDIENTS AND APPEARANCE
MYALEPT
metreleptin injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 76431-210 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Metreleptin (UNII: TL60C27RLH) (Metreleptin - UNII:TL60C27RLH) Metreleptin 11.3 mg in 2.2 mL Inactive Ingredients Ingredient Name Strength Glycine (UNII: TE7660XO1C) Sucrose (UNII: C151H8M554) Polysorbate 20 (UNII: 7T1F30V5YH) Glutamic Acid (UNII: 3KX376GY7L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76431-210-01 1 in 1 CARTON 03/05/2015 1 2.2 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125390 03/05/2015 Labeler - Aegerion Pharmaceuticals, Inc. (613498067)
Trademark Results [Myalept]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MYALEPT 86043958 4607022 Live/Registered |
Amylin Pharmaceuticals, LLC 2013-08-21 |
 MYALEPT 85484675 4589120 Live/Registered |
AEGERION PHARMACEUTICALS, INC. 2011-12-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.