Soothe your skin by PROXIMITY CAPITAL PARTNERS LLC DBA ASUTRA / SOLITEINT Kozmetikum gyarto es forgalmazo, termelo es kereskedelmi Korlatolt Felelossegu Tarsasag Soothe your skin
Soothe your skin by
Drug Labeling and Warnings
Soothe your skin by is a Otc medication manufactured, distributed, or labeled by PROXIMITY CAPITAL PARTNERS LLC DBA ASUTRA, SOLITEINT Kozmetikum gyarto es forgalmazo, termelo es kereskedelmi Korlatolt Felelossegu Tarsasag. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SOOTHE YOUR SKIN- colloidal oatmeal cream
PROXIMITY CAPITAL PARTNERS LLC DBA ASUTRA
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Soothe your skin
Warnings
For external use only. Use only as directed.
Keep away from excessive heat or flame.
When using this product:
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged skin
- do not bandage tightly
Directions
Adults and children 3 months of age and older: Apply as needed.
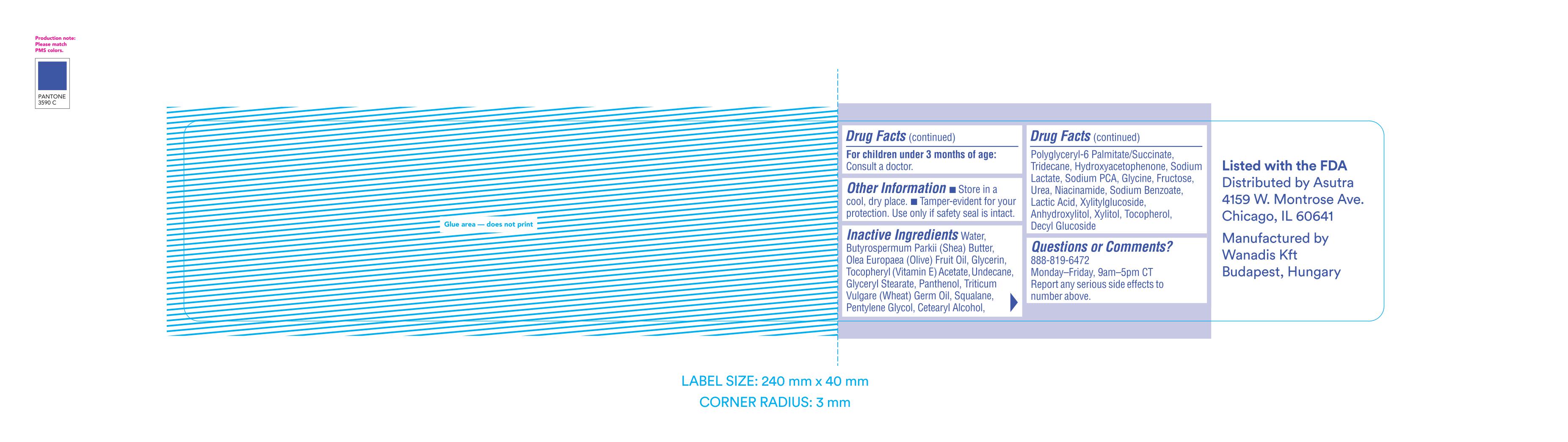
For children under 3 months of age: Consult a doctor.
Other information
- Store in a cool, dry place.
- Tamper-evident for your protection. Use only if safety seal is intact.
Inactive ingredients
Water, Butyrospermum Parkii (Shea) Butter, Olea Europaea (Olive) Fruit Oil, Glycerin, Tocopheryl (Vitamin E) Acetate, Undecane, Glyceryl Stearate, Panthenol, Triticum Vulgare (Wheat) Germ Oil, Squalane, Pentylene Glycol, Cetearyl Alcohol, Polyglyceryl-6 Palmitate/Succinate, Tridecane, Hydroxyacetophenone, Sodium Lactate, Sodium PCA, Glycine, Fructose, Urea, Niacinamide, Sodium Benzoate, Lactic Acid, Xylitylglucoside, Anhydroxylitol, Xylitol, Tocopherol, Decyl Glucoside
Questions or Comments?
888-819-6472
Monday-Friday, 9am-5pm CT
Report any serious side effects to number above.
NDC: 72683-007-01
Asutra*
Soothe your skin
1% Colloidal Oatmeal
Eczema Cream Skin Protectant
3.4 fl oz. | 100 ml.

| SOOTHE YOUR SKIN
colloidal oatmeal cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - PROXIMITY CAPITAL PARTNERS LLC DBA ASUTRA (081214985) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SOLITEINT Kozmetikum gyarto es forgalmazo, termelo es kereskedelmi Korlatolt Felelossegu Tarsasag | 401476254 | manufacture(72683-007) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.