AGAVE CACTUS LIGHT- dimethicone cream
AGAVE CACTUS by
Drug Labeling and Warnings
AGAVE CACTUS by is a Otc medication manufactured, distributed, or labeled by SKINFOOD CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredients:
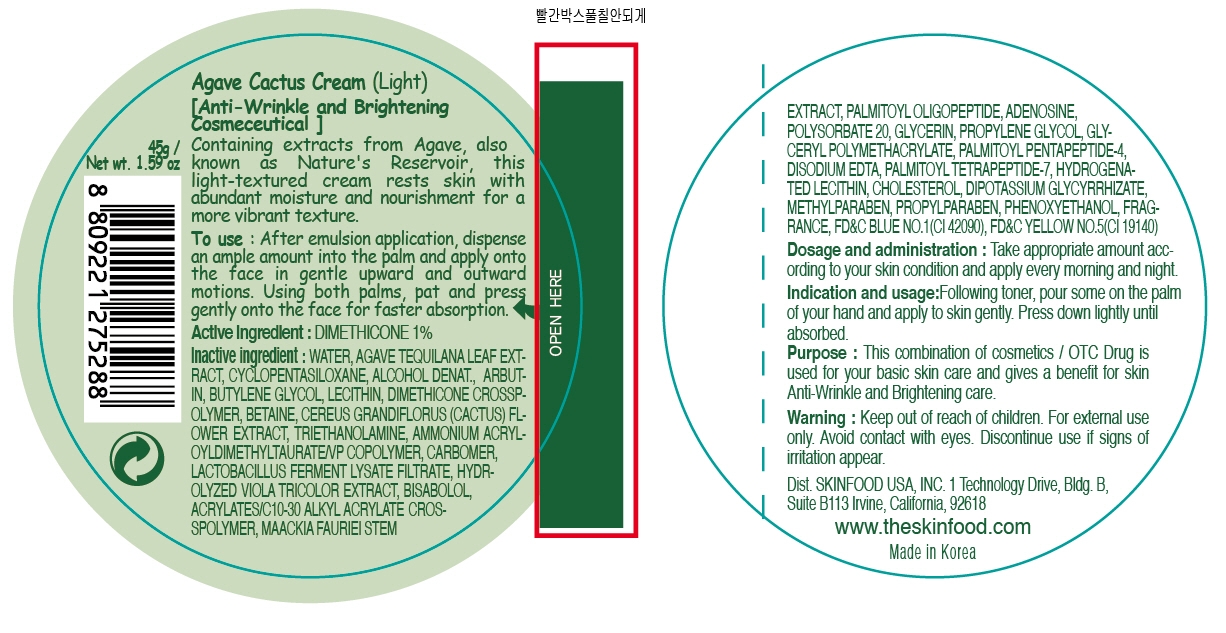
WATER, AGAVE TEQUILANA LEAF EXTRACT, CYCLOPENTASILOXANE, ALCOHOL DENAT., ARBUTIN, BUTYLENE GLYCOL, LECITHIN, DIMETHICONE CROSSPOLYMER, BETAINE, CEREUS GRANDIFLORUS (CACTUS) FLOWER EXTRACT, TRIETHANOLAMINE, AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER, CARBOMER, LACTOBACILLUS FERMENT LYSATE FILTRATE, HYDROLYZED VIOLA TRICOLOR EXTRACT, BISABOLOL, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, MAACKIA FAURIEI STEM EXTRACT, PALMITOYL OLIGOPEPTIDE, ADENOSINE, POLYSORBATE 20, GLYCERIN, PROPYLENE GLYCOL, GLYCERYL POLYMETHACRYLATE, PALMITOYL PENTAPEPTIDE-4, DISODIUM EDTA, PALMITOYL TETRAPEPTIDE-7, HYDROGENATED LECITHIN, CHOLESTEROL, DIPOTASSIUM GLYCYRRHIZATE, METHYLPARABEN, PROPYLPARABEN, PHENOXYETHANOL, FRAGRANCE
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AGAVE CACTUS LIGHT
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 76214-004 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 0.45 g in 45 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) AGAVE TEQUILANA LEAF (UNII: 05545M0E3M) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ARBUTIN (UNII: C5INA23HXF) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) BETAINE (UNII: 3SCV180C9W) PHENOXYETHANOL (UNII: HIE492ZZ3T) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) MAACKIA FLORIBUNDA STEM (UNII: 84UXW52K8Y) PALMITOYL OLIGOPEPTIDE (UNII: HO4ZT5S86C) ADENOSINE (UNII: K72T3FS567) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYSORBATE 20 (UNII: 7T1F30V5YH) GLYCERIN (UNII: PDC6A3C0OX) EDETATE DISODIUM (UNII: 7FLD91C86K) PALMITOYL PENTAPEPTIDE-4 (UNII: KK181SM5JG) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) CHOLESTEROL (UNII: 97C5T2UQ7J) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76214-004-01 45 g in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 03/01/2011 Labeler - SKINFOOD CO., LTD. (690324173) Registrant - SKINFOOD CO., LTD. (690324173) Establishment Name Address ID/FEI Business Operations SKINFOOD CO., LTD. 690324173 manufacture
Trademark Results [AGAVE CACTUS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AGAVE CACTUS 88122131 not registered Dead/Abandoned |
Bronco Wine Company 2018-09-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.