ELUCIREM- gadopiclenol injection
ELUCIREM by
Drug Labeling and Warnings
ELUCIREM by is a Prescription medication manufactured, distributed, or labeled by Guerbet LLC, Liebel-Flarsheim Company LLC, Guerbet. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ELUCIREMTM safely and effectively. See full prescribing information for ELUCIREM.
ELUCIREMTM (gadopiclenol) injection, for intravenous use
Initial U.S. Approval: 2022WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
See full prescribing information for complete boxed warning
- Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. ELUCIREM is not approved for intrathecal use. (5.1)
-
GBCAs increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of ELUCIREM in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. The risk for NSF appears highest among patients with:
- Chronic, severe kidney disease (GFR <30 mL/min/1.73 m2), or
- Acute kidney injury.
Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (for example, age >60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing. (5.2)
RECENT MAJOR CHANGES
Dosage and Administration
Directions for Use of Imaging Bulk Package (2.5) 11/2025INDICATIONS AND USAGE
ELUCIREM is a gadolinium-based contrast agent indicated in adult and pediatric patients aged 2 years and older for use with magnetic resonance imaging (MRI) to detect and visualize lesions with abnormal vascularity in: (1)
- the central nervous system (brain, spine, and associated tissues),
- the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system). (1)
DOSAGE AND ADMINISTRATION
- The recommended dose for adult and pediatric patients aged 2 years and older is 0.05 mmol/kg actual body weight (equivalent to 0.1 mL/kg) administered intravenously at approximately 2 mL/sec. (2.1)
DOSAGE FORMS AND STRENGTHS
Injection: 0.5 mmol/mL of gadopiclenol in single-dose vials, single-dose prefilled syringes, pharmacy bulk packages, and imaging packages (3)
CONTRAINDICATIONS
History of hypersensitivity reactions to ELUCIREM (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (incidence >0.2%) in patients who received ELUCIREM are injection site pain, headache, nausea, injection site warmth and coldness, dizziness, and localized swelling. (6.1)
To report SUSPECTED ADVERSE REACTIONS contact GUERBET LLC at 1-877-729-6679 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS
Pregnancy: Use only if imaging is essential during pregnancy and cannot be delayed. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration and Imaging Instructions
2.3 Directions for Use of Single-Dose Vial and Pre-filled Syringe
2.4 Directions for Use of Pharmacy Bulk Package
2.5 Directions for Use of Imaging Bulk Package
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk Associated with Intrathecal Use
5.2 Nephrogenic Systemic Fibrosis
5.3 Hypersensitivity Reactions
5.4 Gadolinium Retention
5.5 Acute Kidney Injury
5.6 Extravasation and Injection Site Reactions
5.7 Interference with Visualization of Lesions Visible with Non-Contrast MRI
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies
14.2 Visualization of CNS Lesions
14.3 Visualization of Body Lesions
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISK ASSOCIATED WITH INTRATHECAL USE and NEPHROGENIC SYSTEMIC FIBROSIS
Risk Associated with Intrathecal Use
Intrathecal administration of gadolinium-based contrast agents (GBCAs) can cause serious adverse reactions including death, coma, encephalopathy, and seizures. ELUCIREM is not approved for intrathecal use [see Warnings and Precautions (5.1)].Nephrogenic Systemic Fibrosis
GBCAs increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of ELUCIREM in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.
The risk for NSF appears highest among patients with:- Chronic, severe kidney disease (GFR < 30 mL/min/1.73 m2), or
- Acute kidney injury.
Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension, diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
For patients at highest risk for NSF, do not exceed the recommended ELUCIREM dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
ELUCIREMTM is indicated in adult and pediatric patients aged 2 years and older for use with magnetic resonance imaging (MRI) to detect and visualize lesions with abnormal vascularity in:
- the central nervous system (brain, spine, and associated tissues),
- the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system).
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of ELUCIREM for adult and pediatric patients aged 2 years and older is 0.05 mmol/kg actual body weight (equivalent to 0.1 mL/kg) administered intravenously at approximately 2 mL/sec.
2.2 Administration and Imaging Instructions
- Use aseptic technique for all handling and administration of ELUCIREM.
- Visually inspect ELUCIREM for particulate matter and discoloration prior to administration. Do not use the solution if any particulate matter is present or the solution is discolored.
- Do not mix with other medications because of the potential for chemical incompatibility.
- Prime intravenous line before use.
- Administer ELUCIREM as an intravenous bolus injection, manually or by compatible power injector. The recommended injection rate is approximately 2 mL/second.
- Flush the intravenous line with 0.9% Sodium Chloride Injection, USP after the administration of ELUCIREM.
- Contrast MRI can begin immediately following the injection of ELUCIREM.
2.3 Directions for Use of Single-Dose Vial and Pre-filled Syringe
Vial
- Do not pierce the rubber stopper more than once.
- Aseptically draw up ELUCIREM into a disposable syringe and use immediately.
- If solidification occurs in the vial because of exposure to the cold, bring the vial of ELUCIREM to room temperature before use and inspect that the solution is clear, colorless to yellow without any particulate matter and discoloration.
- Discard any unused portion.
Pre-filled syringe
- Remove the tip cap of the syringe, screw the plunger rod and use immediately.
- All luer connections should be gently hand tightened without over tightening, to ensure secure connections and to prevent damage to the device.
- Pre-filled syringes must not be frozen. Frozen pre-filled syringes of ELUCIREM should be discarded.
- Discard any unused portion.
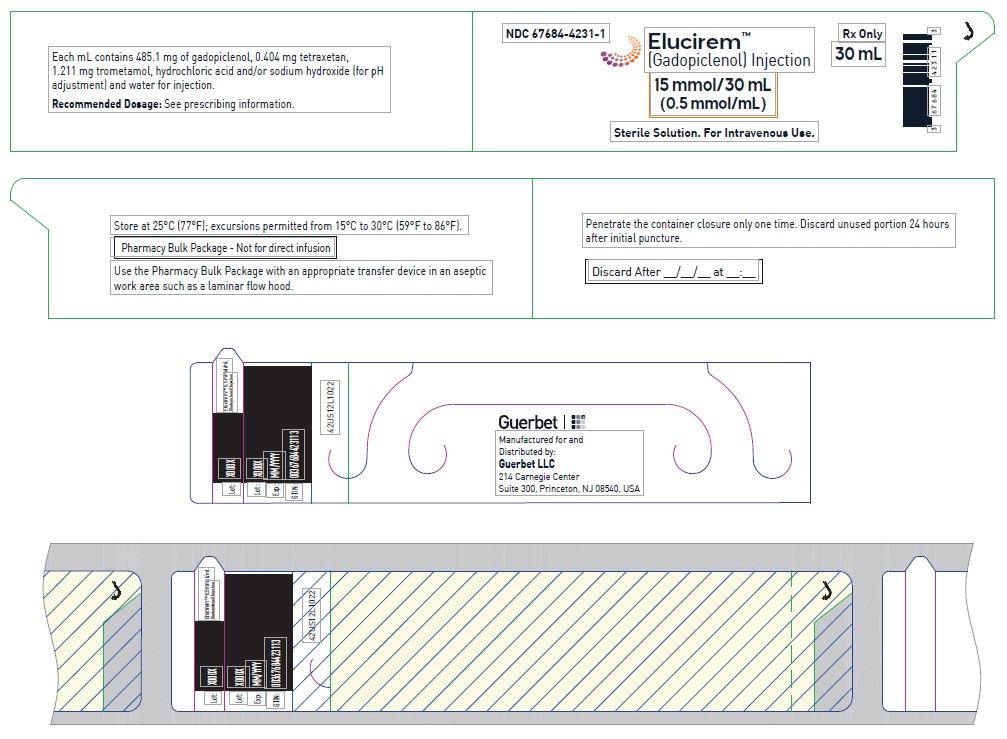
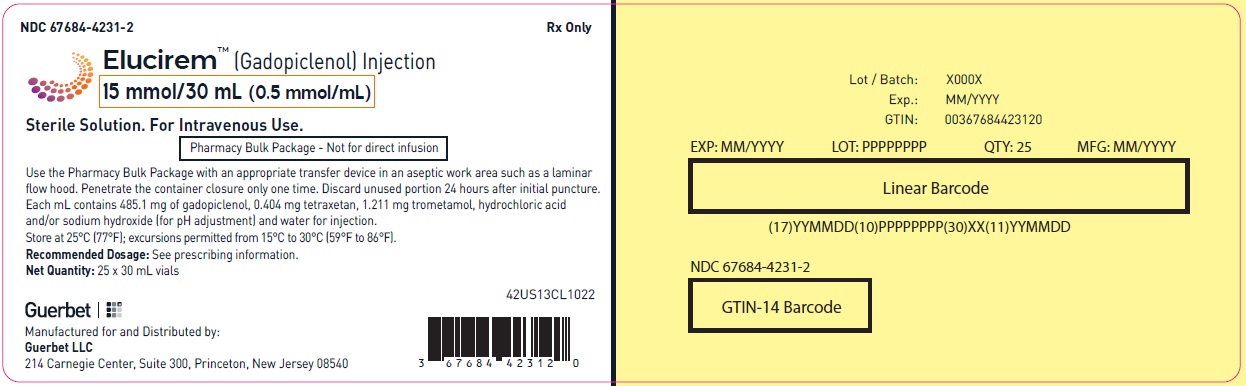
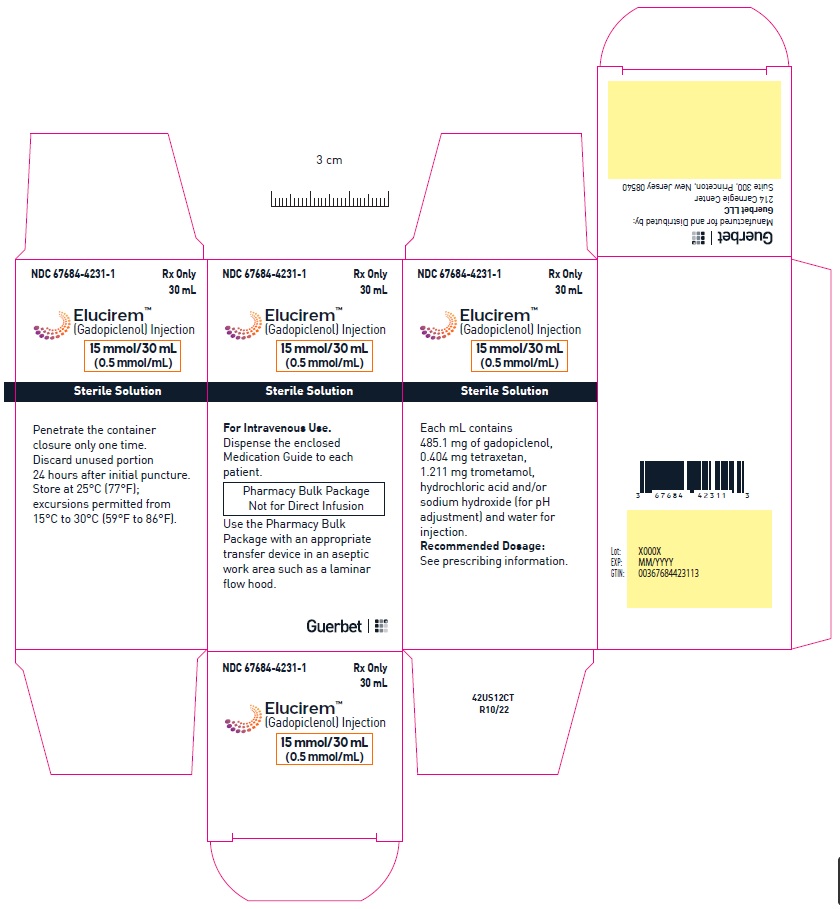
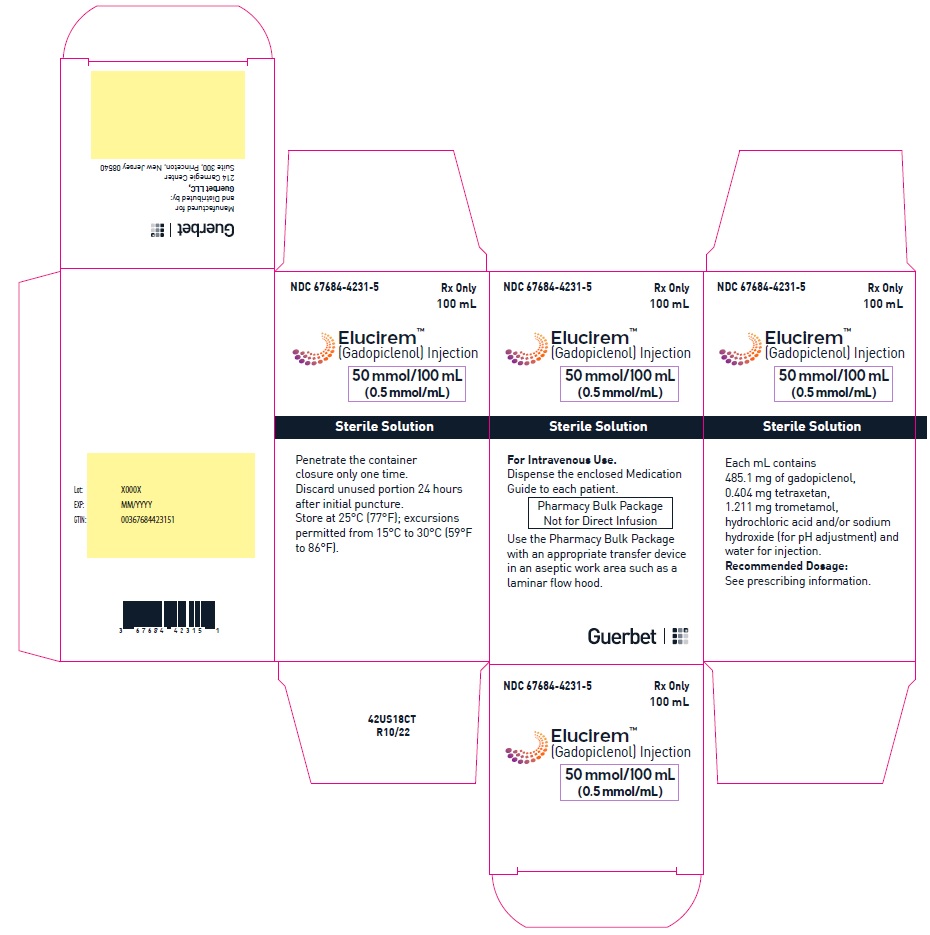
2.4 Directions for Use of Pharmacy Bulk Package
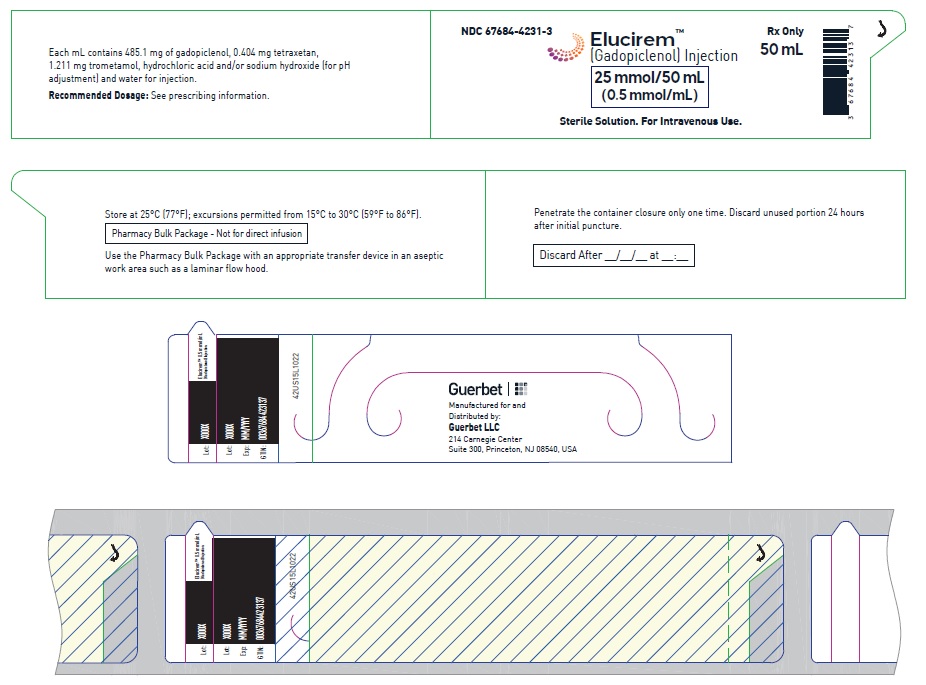
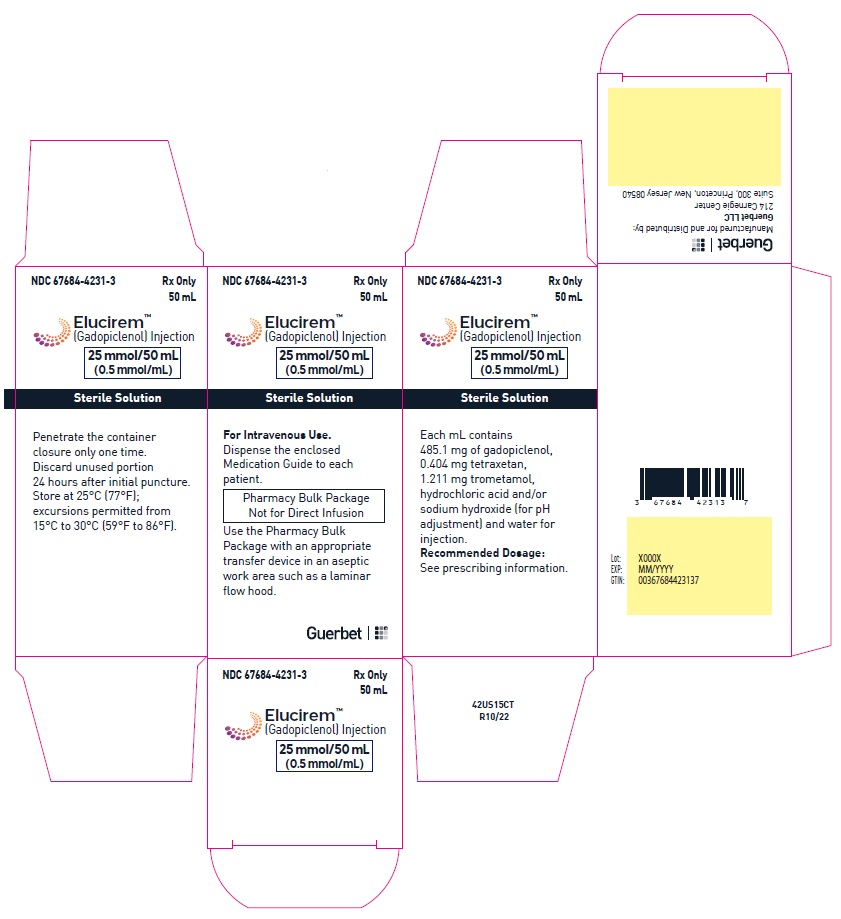
- Do not use the Pharmacy Bulk Package for direct infusion.
- Perform the transfer of ELUCIREM from the Pharmacy Bulk Package in an aseptic work area, such as laminar flow hood, using aseptic technique and suitable transfer device for filling empty syringes.
- Penetrate the closure only one time. Once the container closure is punctured, do not remove the Pharmacy Bulk Package from the aseptic work area.
- The Pharmacy Bulk Package is used with an appropriate transfer device for filling empty sterile syringes. Use each individual dose of ELUCIREM promptly following withdrawal from the Pharmacy Bulk Package.
- Use the contents of the Pharmacy Bulk Package within 24 hours at room temperature after initial puncture.
- If solidification occurs in the vial because of exposure to the cold, bring the vial of ELUCIREM to room temperature before use and inspect that the solution is clear, colorless to yellow without any particulate matter and discoloration.
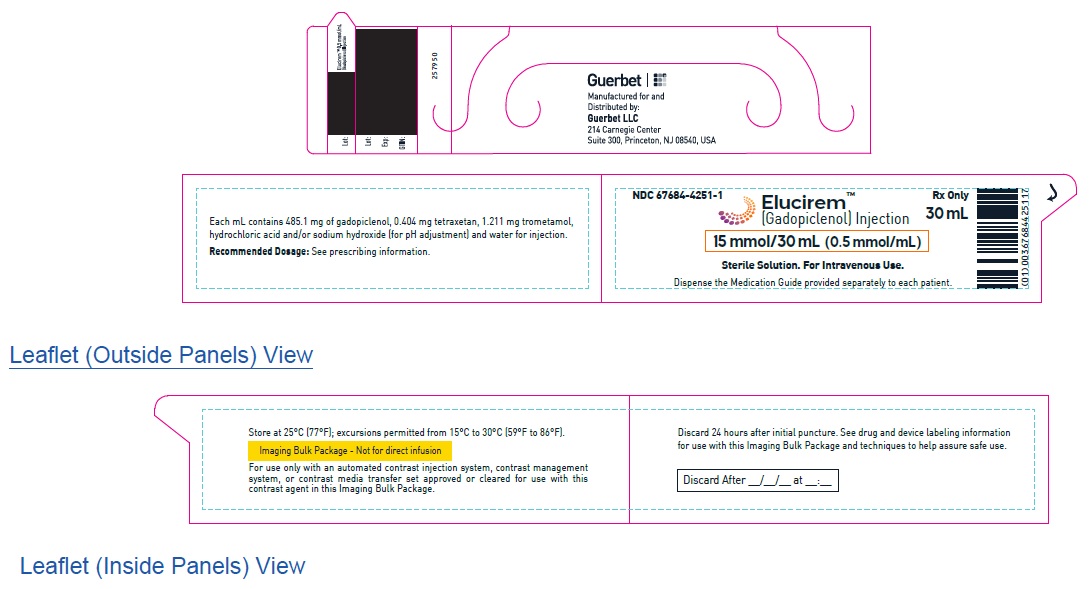
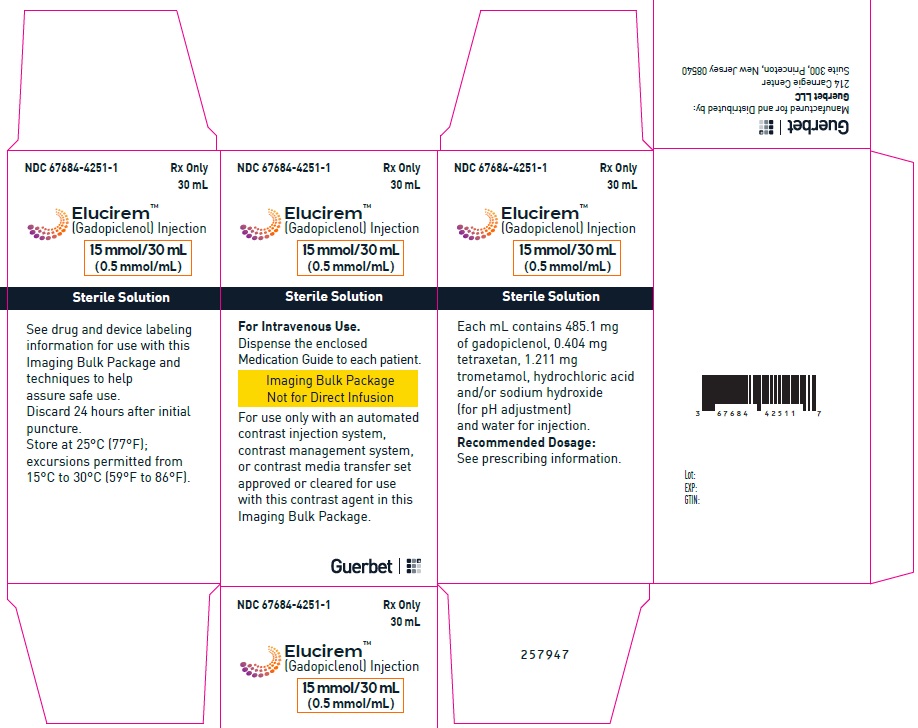
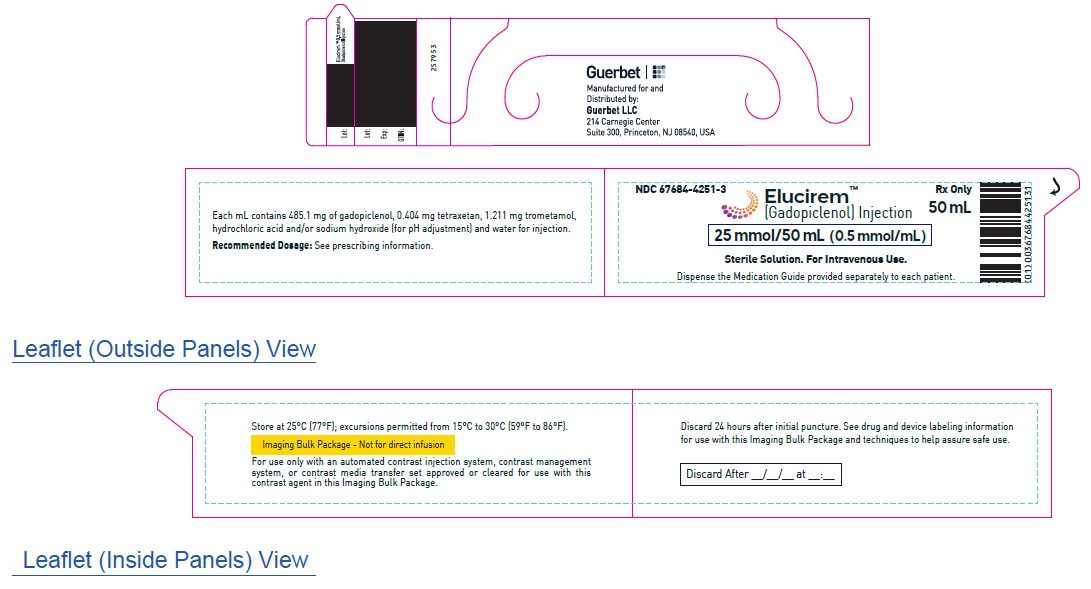
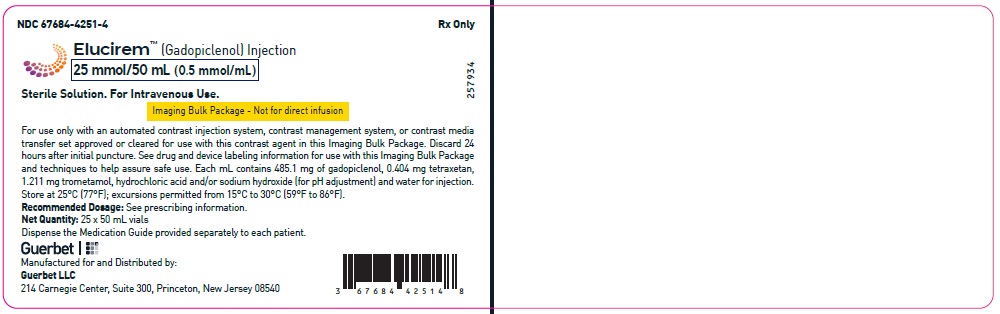
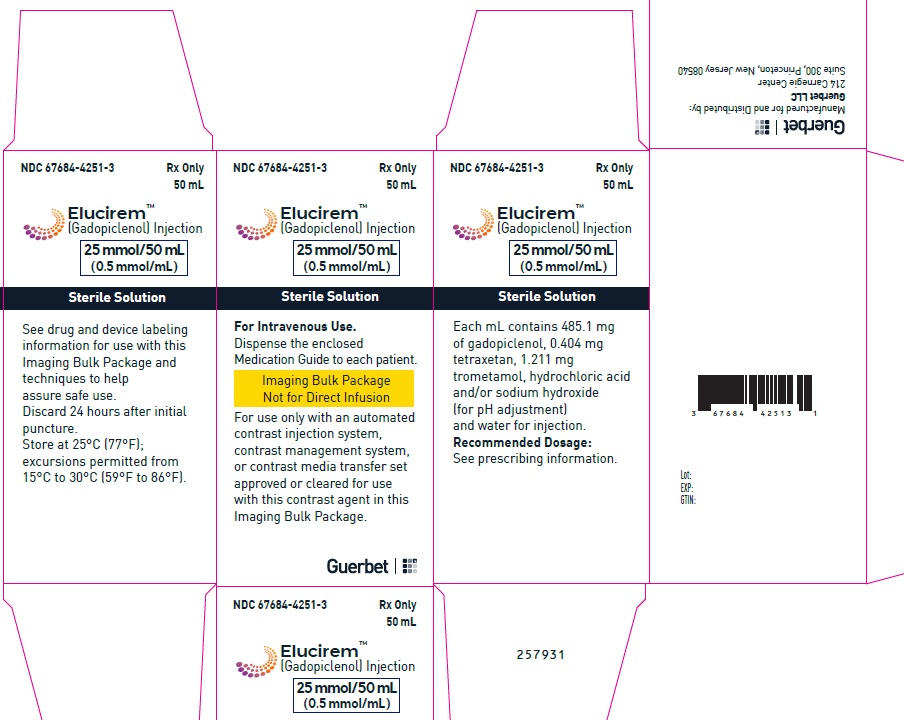
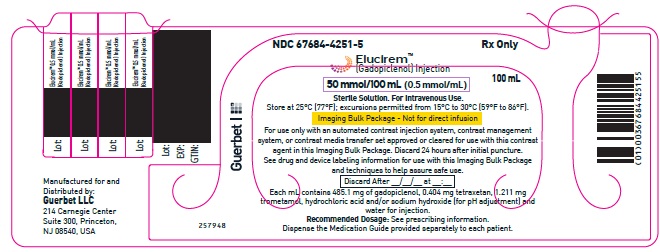
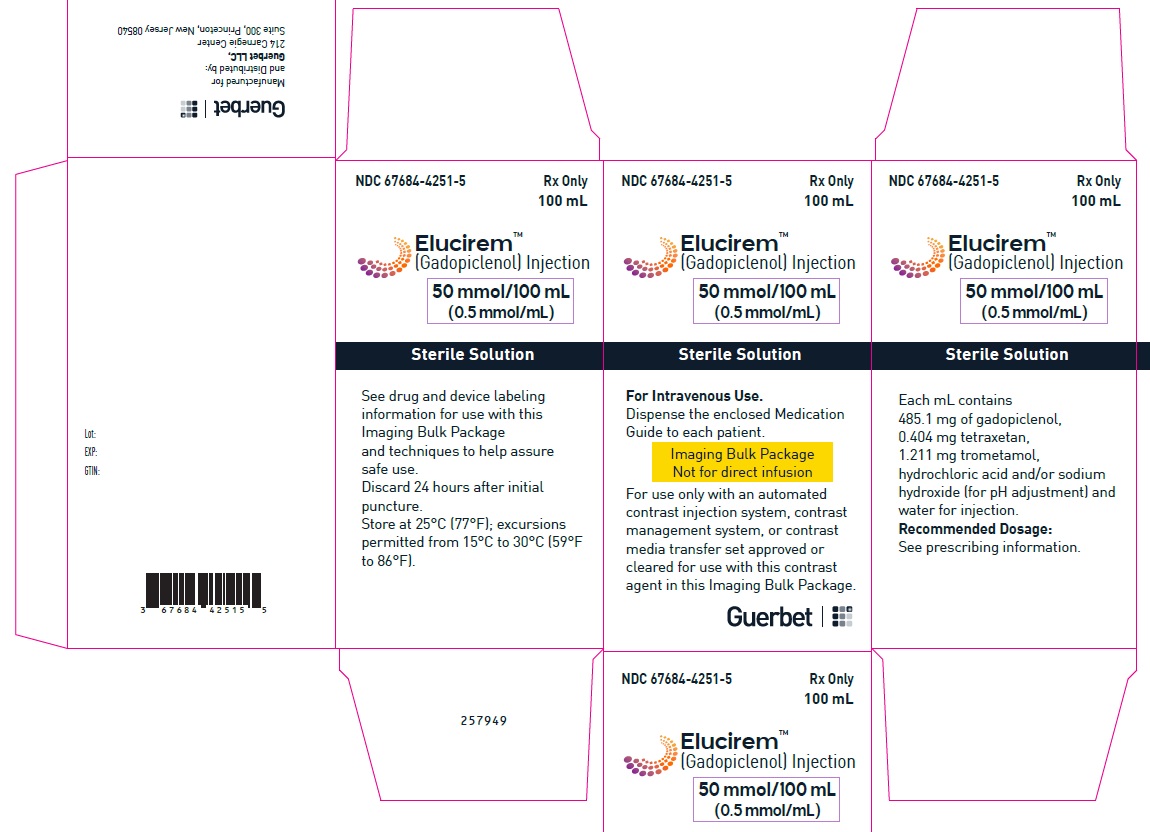
2.5 Directions for Use of Imaging Bulk Package
- ELUCIREM Imaging Bulk Package (IBP) is for intravenous use and not for direct infusion.
- The IBP is a container of a sterile preparation for parenteral use that contains multiple single doses of ELUCIREM for multiple patients for use with an automated contrast injection system, contrast management system, or contrast media transfer set approved or cleared for use with this contrast agent in this IBP.
- See drug and device labeling for information on devices indicated for use with this IBP and techniques to help assure safe use.
- The ELUCIREM IBP is to be used only in a room designated to perform radiological procedures that involve administration of a contrast agent.
- Utilize aseptic technique for penetrating the container closure of the IBP and transferring ELUCIREM.
- Penetrate the container closure only one time with a suitable sterile component of the automated contrast injection system, contrast management system, or contrast media transfer set (e.g., transfer spike) approved or cleared for use with this IBP.
- During the entire period of use, ensure that the contents of the ELUCIREM IBP container are in continuous contact with the automated contrast injector system, contrast management system or contrast media transfer set. Do not remove the dispensing set from the IBP container closure to ensure that the protection of the contrast media against any possible contamination.
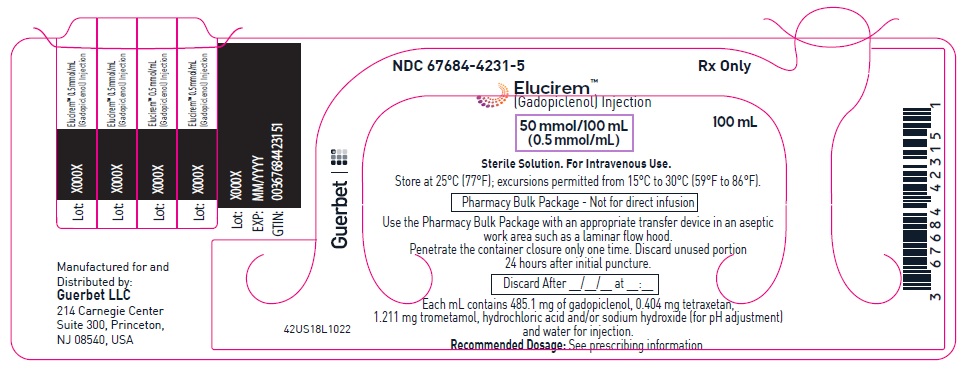
- Once the ELUCIREM IBP container is punctured, do not remove it from the work area. Store the ELUCIREM IBP at 25° C (77° F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
- A maximum use time from initial puncture is 24 hours. Discard any unused ELUCIREM 24 hours after initial puncture of the IBP.
- After the container closure is punctured, if the integrity of IBP and the delivery system cannot be assured through direct continuous supervision, the IBP and all associated disposables for the automated contrast injection system, contrast management system, or contrast media transfer set should be discarded.
- If solidification occurs in the IBP because of exposure to the cold, bring the IBP container of ELUCIREM to room temperature, before use and inspect that the solution is clear, colorless to yellow without any particulate matter and discoloration.
-
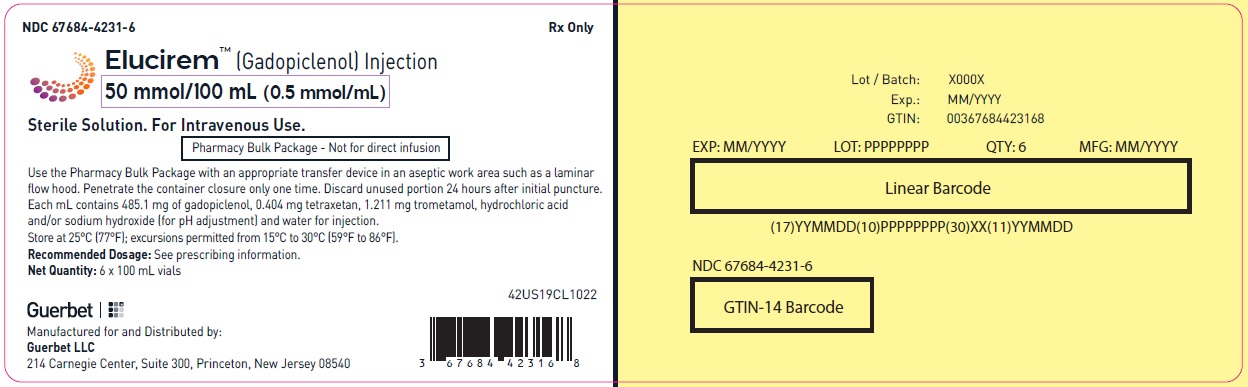
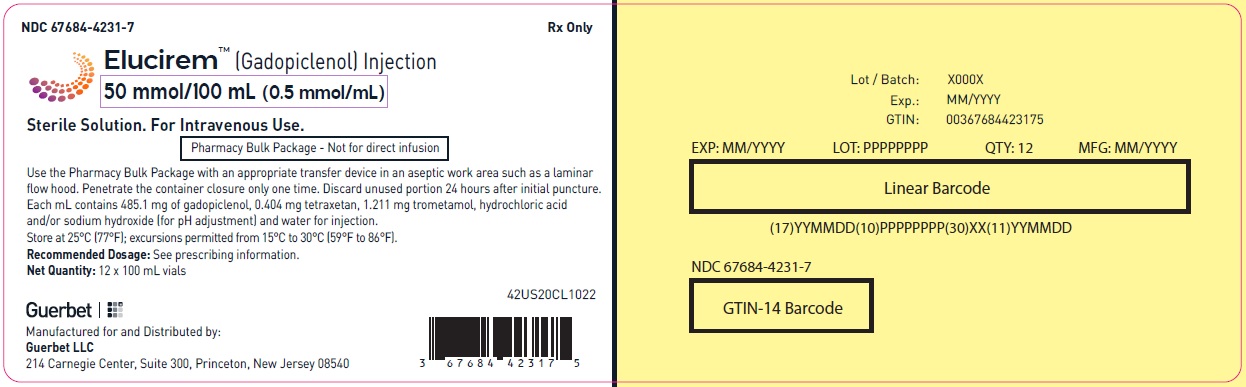
3 DOSAGE FORMS AND STRENGTHS
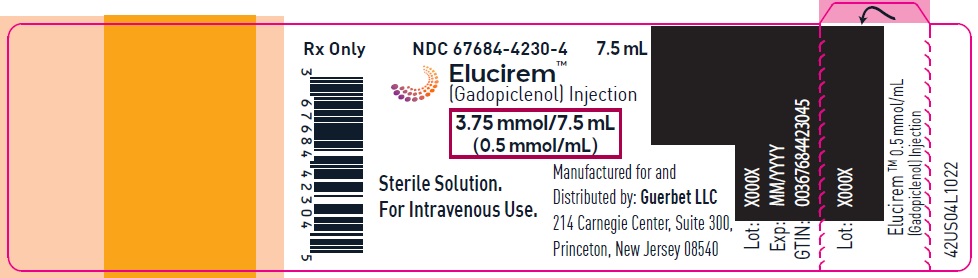
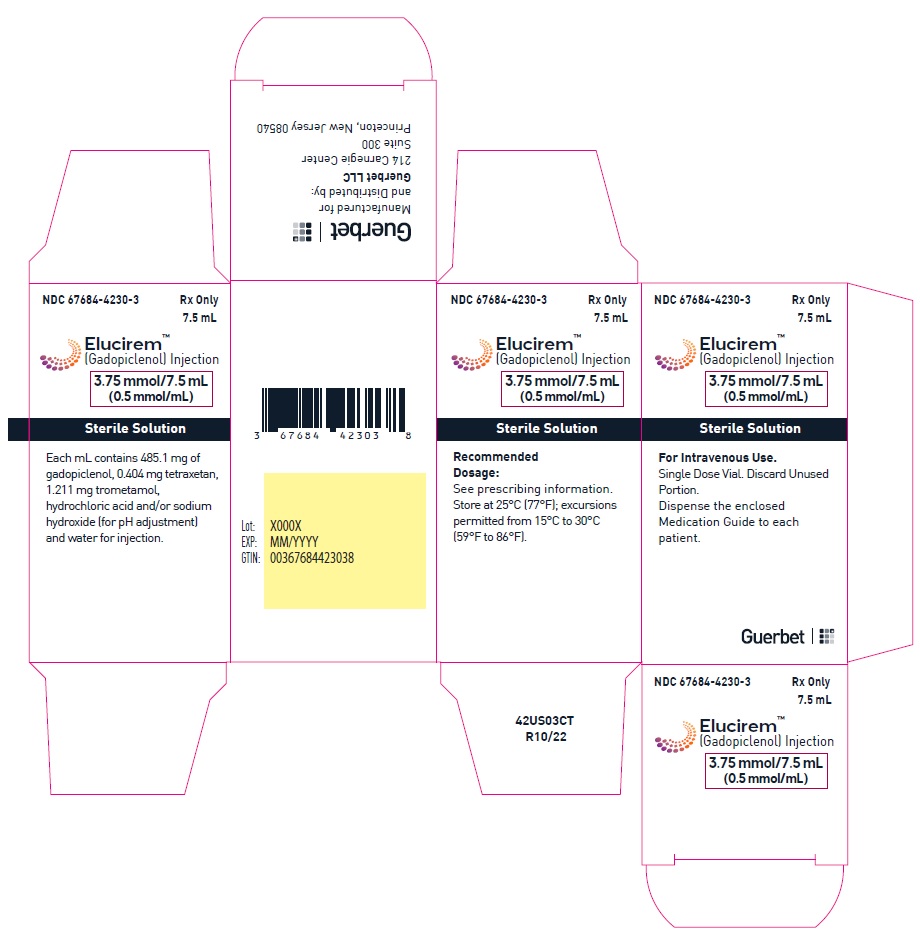
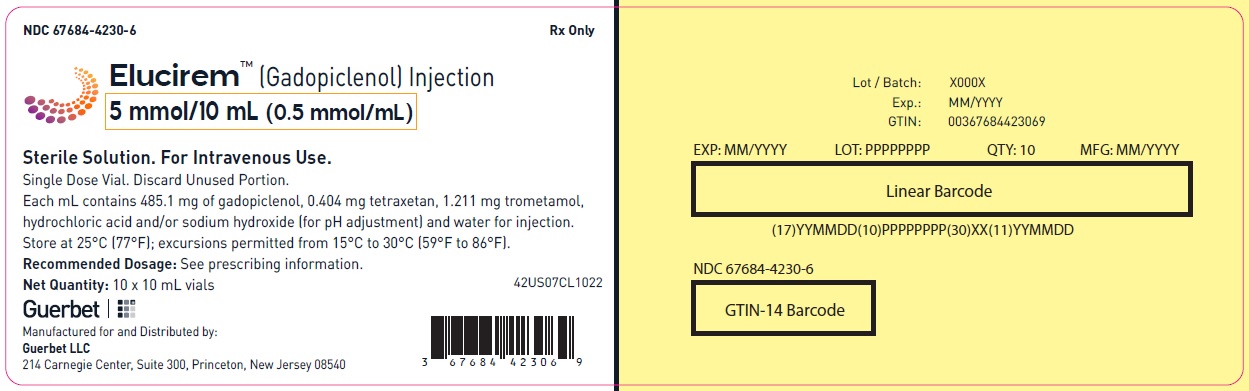
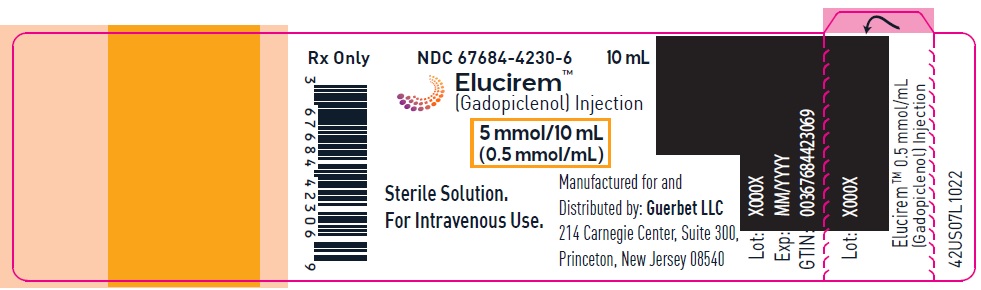
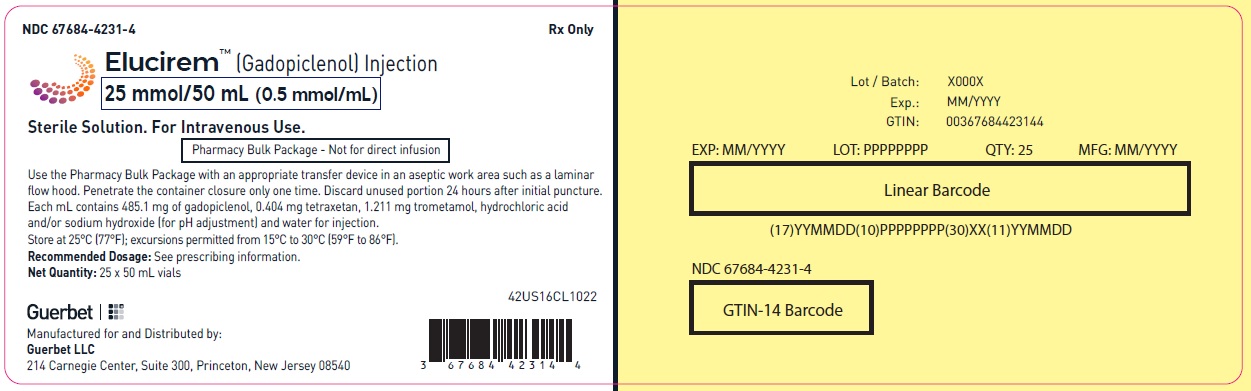
Injection: ELUCIREM is a clear, colorless to yellow aqueous solution at a concentration of 0.5 mmol/mL of gadopiclenol available as:
Strength
Packaging
- 1.5 mmol/3 mL (0.5 mmol/mL)
- 3.75 mmol/7.5 mL (0.5 mmol/mL)
- 5 mmol/10 mL (0.5 mmol/mL)
- 7.5 mmol/15 mL (0.5 mmol/mL)
Single-dose vials (glass)- 3.75 mmol/7.5 mL (0.5 mmol/mL)
- 5 mmol/10 mL (0.5 mmol/mL)
- 7.5 mmol/15 mL (0.5 mmol/mL)
Single-dose prefilled syringes (plastic)- 15 mmol/30 mL (0.5 mmol/mL)
- 25 mmol/50 mL (0.5 mmol/mL)
- 50 mmol/100 mL (0.5 mmol/mL)
Pharmacy bulk package (glass)- 15 mmol/30 mL (0.5 mmol/mL)
- 25 mmol/50 mL (0.5 mmol/mL)
- 50 mmol/100 mL (0.5 mmol/mL)
Imaging Bulk Package (glass) - 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk Associated with Intrathecal Use
Intrathecal administration of GBCAs can cause serious adverse reactions including death, coma, encephalopathy, and seizures. The safety and effectiveness of ELUCIREM have not been established with intrathecal use. ELUCIREM is not approved for intrathecal use [see Dosage and
Administration (2.1)].5.2 Nephrogenic Systemic Fibrosis
GBCAs increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of ELUCIREM among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR <30 mL/min/1.73 m2) as well as patients with acute kidney injury. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30-59 mL/min/1.73 m2) and little, if any, for patients with chronic, mild kidney disease (GFR 60-89 mL/min/1.73 m2). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle, and internal organs. Report any diagnosis of NSF following ELUCIREM administration to Guerbet LLC (1-877-729-6679) or FDA (1-800-FDA-1088 or www.fda.gov/medwatch).
Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronically reduced renal function (e.g., age >60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing.
Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and the degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. For patients at highest risk for NSF, do not exceed the recommended ELUCIREM dose and allow a sufficient period of time for elimination of the drug prior to re-administration. For patients receiving hemodialysis, physicians may consider the prompt initiation of hemodialysis following the administration of a GBCA in order to enhance the contrast agent’s elimination [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. The usefulness of hemodialysis in the prevention of NSF is unknown.
5.3 Hypersensitivity Reactions
With GBCAs, serious hypersensitivity reactions have occurred. In most cases, initial symptoms occurred within minutes of GBCA administration and resolved with prompt emergency treatment.
- Before ELUCIREM administration, assess all patients for any history of a reaction to contrast media, bronchial asthma and/or allergic disorders. These patients may have an increased risk for a hypersensitivity reaction to ELUCIREM.
- ELUCIREM is contraindicated in patients with history of hypersensitivity reactions to ELUCIREM [see Contraindications (4)].
- Administer ELUCIREM only in situations where trained personnel and therapies are promptly available for the treatment of hypersensitivity reactions, including personnel trained in resuscitation.
- During and following ELUCIREM administration, observe patients for signs and symptoms of hypersensitivity reactions.
5.4 Gadolinium Retention
Gadolinium is retained for months or years in several organs. The highest concentrations (nanomoles per gram of tissue) have been identified in the bone, followed by other organs (e.g. brain, skin, kidney, liver, and spleen). The duration of retention also varies by tissue and is longest in bone. Linear GBCAs cause more retention than macrocyclic GBCAs. At equivalent doses, gadolinium retention varies among the linear agents with gadodiamide causing greater retention than other linear agents such as gadoxetate disodium and gadobenate dimeglumine. Retention is lowest and similar among the macrocyclic GBCAs such as gadoterate meglumine, gadobutrol, gadoteridol, and gadopiclenol.
Consequences of gadolinium retention in the brain have not been established. Pathologic and clinical consequences of GBCA administration and retention in skin and other organs have been established in patients with impaired renal function [see Warnings and Precautions (5.2)]. There are rare reports of pathologic skin changes in patients with normal renal function. Adverse events involving multiple organ systems have been reported in patients with normal renal function without an established causal link to gadolinium.
While clinical consequences of gadolinium retention have not been established in patients with normal renal function, certain patients might be at higher risk. These include patients requiring multiple lifetime doses, pregnant and pediatric patients, and patients with inflammatory conditions. Consider the retention characteristics of the agent when choosing a GBCA for these patients. Minimize repetitive GBCA imaging studies, particularly closely spaced studies, when possible.
5.5 Acute Kidney Injury
In patients with chronically reduced renal function, acute kidney injury requiring dialysis has occurred with the use of GBCAs. The risk of acute kidney injury may increase with increasing dose of the contrast agent. Do not exceed the recommended dose.
5.6 Extravasation and Injection Site Reactions
Injection site reactions such as injection site pain have been reported in the clinical studies with ELUCIREM [see Adverse Reactions (6.1)]. Extravasation during ELUCIREM administration may result in tissue irritation [see Nonclinical Toxicology (13.2)]. Ensure catheter and venous patency before the injection of ELUCIREM.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed elsewhere in labeling:
- Nephrogenic Systemic Fibrosis [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Contraindications (4) and Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of ELUCIREM was evaluated in 1,047 patients who received ELUCIREM at doses ranging from 0.025 mmol/kg (one half the recommended dose) to 0.3 mmol/kg (six times the recommended dose). A total of 708 patients received the recommended dose of 0.05 mmol/kg. Among patients who received the recommended dose, the average age was 51 years (range 2 years to 88 years) and 56% were female. The ethnic distribution was 79% White, 10% Asian, 7% American Indian or Alaska native, 2% Black, and 2% patients of other or unspecified ethnic groups.
Overall, approximately 4.7% of subjects receiving the labeled dose reported one or more adverse reactions.
Table 1 lists adverse reactions that occurred in > 0.2% of patients who received 0.05 mmol/kg ELUCIREM.
Table 1. Adverse Reactions Reported in > 0.2% of Patients Receiving ELUCIREM in Clinical Trials Adverse Reaction
ELUCIREM 0.05 mmol/kg
(n=708)
(%)Injection site pain
0.7
Headache
0.7
Nausea
0.4
Injection site warmth
0.4
Injection site coldness
0.3
Dizziness
0.3
Localized swelling
0.3
Adverse reactions that occurred with a frequency ≤ 0.2% in patients who received 0.05 mmol/kg ELUCIREM included: maculopapular rash, vomiting, worsened renal impairment, feeling hot, pyrexia, oral paresthesia, dysgeusia, diarrhea, pruritus, allergic dermatitis, erythema, injection site paresthesia, Cystatin C increase, and blood creatinine increase.
Adverse Reactions in Pediatric Patients
One study with a single dose of ELUCIREM (0.05 mmol/kg) was conducted in 80 pediatric patients aged 2 years to 17 years, including 60 patients who underwent a central nervous system (CNS) MRI and 20 patients who underwent a body MRI. One adverse reaction (maculopapular rash of moderate severity) in one patient (1.3%) was reported in the CNS cohort.6.2 Postmarketing Experience
The following additional adverse reactions have been identified during postmarketing use of ELUCIREM or other GBCAs. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: Acute pancreatitis with onset within 48 hours after GBCA administration.
Respiratory, Thoracic and Mediastinal Disorders: Acute respiratory distress syndrome, pulmonary edema.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on ELUCIREM use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. GBCAs cross the human placenta and result in fetal exposure and gadolinium retention. The available human data on GBCA exposure during pregnancy and adverse fetal outcomes are limited and inconclusive (see Data).In animal reproduction studies, there were no adverse developmental effects observed in rats or rabbits with intravenous administration of ELUCIREM during organogenesis (see Data). Because of the potential risks of gadolinium to the fetus, use ELUCIREM only if imaging is essential during pregnancy and cannot be delayed.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20% respectively.
Data
Human Data
Contrast enhancement is visualized in the placenta and fetal tissues after maternal GBCA administration.Cohort studies and case reports on exposure to GBCAs during pregnancy have not reported a clear association between GBCAs and adverse effects in the exposed neonates. However, a retrospective cohort study comparing pregnant women who had a GBCA MRI to pregnant women who did not have an MRI reported a higher occurrence of stillbirths and neonatal deaths in the group receiving GBCA MRI. Limitations of this study include a lack of comparison with non-contrast MRI and lack of information about the maternal indication for MRI. Overall, these data preclude a reliable evaluation of the potential risk of adverse fetal outcomes with the use of GBCAs in pregnancy.
Animal Data
Gadolinium Retention: GBCAs administered to pregnant non-human primates (0.1 mmol/kg on gestational days 85 and 135) result in measurable gadolinium concentration in the offspring in bone, brain, skin, liver, kidney, and spleen for at least 7 months. GBCAs administered to pregnant mice (2 mmol/kg daily on gestational days 16 through 19) result in measurable gadolinium concentrations in the pups in bone, brain, kidney, liver, blood, muscle, and spleen at one-month postnatal age.Reproductive Toxicology: Animal reproduction studies conducted with gadopiclenol showed some signs of maternal toxicity in rats at 10 mmol/kg and rabbits at 5 mmol/kg (corresponding to 52 times and 57 times the recommended human dose, respectively). This maternal toxicity was characterized in both species by swelling, decreased activity, and lower gestation weight gain and food consumption.
No effect on embryo-fetal development was observed in rats at 10 mmol/kg (corresponding to 52 times the recommended human dose). In rabbits, a lower mean fetal body weight was observed at 5 mmol/kg (corresponding to 57 times the recommended human dose) and this was attributed as a consequence of the lower gestation weight gain.
8.2 Lactation
Risk Summary
There are no data on the presence of gadopiclenol in human milk, the effects on the breastfed infant, or the effects on milk production. However, published lactation data on other GBCAs indicate that 0.01% to 0.04% of the maternal gadolinium dose is excreted in breast milk. Additionally, there is limited GBCA gastrointestinal absorption in the breast-fed infant. Gadopiclenol is present in rat milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ELUCIREM and any potential adverse effects on the breastfed infant from ELUCIREM or from the underlying maternal condition.Data
In lactating rats receiving single intravenous injection of [153Gd]-gadopiclenol, 0.3% and 0.2% of the total administered radioactivity was transferred to the pups via maternal milk at 6 hours and 24 hours after administration, respectively. Furthermore, in nursing rat pups, oral absorption of gadopiclenol was 3.6%.8.4 Pediatric Use
The safety and effectiveness of ELUCIREM for use with MRI to detect and visualize lesions with abnormal vascularity in the CNS (brain, spine, and associated tissues), and the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system) have been established in pediatric patients aged 2 years and older.
Use of ELUCIREM in this age group is supported by evidence from adequate and well-controlled studies in adults with additional pharmacokinetic and safety data from an open-label, uncontrolled, multicenter, single dose study of ELUCIREM (0.05 mmol/kg) in 80 pediatric patients aged 2 to 17 years. The 80 patients consisted of 60 patients who underwent a CNS MRI and 20 patients who underwent a body MRI [see Adverse Reactions (6.1) and Clinical Pharmacology (12.3)].
The safety and effectiveness of ELUCIREM have not been established in pediatric patients younger than 2 years of age.
8.5 Geriatric Use
Of the total number of ELUCIREM-treated patients in clinical studies, 270 (26%) patients were 65 years of age and over, while 62 (6%) patients were 75 years of age and over. No overall differences in safety or efficacy were observed between these subjects and younger subjects.
This drug is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function.
8.6 Renal impairment
In patients with renal impairment, the exposure of gadopiclenol is increased compared to patients with normal renal function. This may increase the risk of adverse reactions such as nephrogenic systemic fibrosis (NSF). Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities. No dose adjustment of ELUCIREM is recommended for patients with renal impairment. ELUCIREM can be removed from the body by hemodialysis [see Warnings and Precautions (5.2, 5.4, 5.5) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Among subjects who received a single 0.3 mmol/kg intravenous dose of gadopiclenol (6 times the recommended dose of ELUCIREM), headache and nausea were the most frequently reported adverse reactions. Gadopiclenol can be removed from the body by hemodialysis [see Clinical Pharmacology (12.3)].
-
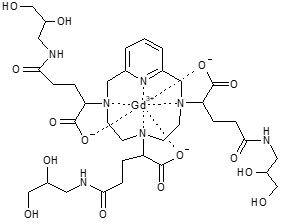
11 DESCRIPTION
ELUCIREM is a gadolinium-based contrast agent, which contains gadopiclenol, a paramagnetic macrocyclic non-ionic complex of gadolinium.
The chemical name for gadopiclenol is rac-[(2R,2'Ξ,2''Ξ)-2,2',2''-(3,6,9-triaza-κ3N3,N6,N9-1(2,6)-pyridina-κN1-cyclodecaphane-3,6,9-triyl)tris(5-{[(2Ξ)-2,3-dihydroxypropyl]amino}-5-oxopentanoato-κ3O1,O1',O1'')(3−)]gadolinium with a molecular weight of 970.11 g/mol and a molecular formula of 970.11 g/mol and a molecular formula of C35H54GdN7O15
ELUCIREM is a sterile, nonpyrogenic, clear, colorless to yellow aqueous solution for intravenous use.
Each mL contains 485.1 mg of gadopiclenol (equivalent to 0.5 mmol of gadopiclenol and 78.6 mg of gadolinium) and the following inactive ingredients: 0.404 mg tetraxetan, 1.211 mg trometamol, hydrochloric acid and/or sodium hydroxide (for pH adjustment, if needed), and water for injection.
The main physicochemical properties of ELUCIREM are provided in Table 2.
Table 2. Physicochemical properties of ELUCIREM Parameter
Value
Density at 20°C
1.211 g/cm3
Mean viscosity at 20°C
12.6 mPa.s
Mean viscosity at 37°C
7.6 mPa.s
Osmolality at 37°C
850 mOsm/kg water
pH
7.0 – 7.8
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Gadopiclenol is a paramagnetic molecule (macrocyclic non-ionic complex of gadolinium) that develops a magnetic moment when placed in a magnetic field. The magnetic moment alters the relaxation rates of water protons in its vicinity in the body, leading to an increase in signal intensity (brightness) of tissues.
12.2 Pharmacodynamics
In MRI, visualization of normal and pathological tissue depends in part on variations in the radiofrequency signal intensity that occur with:
- differences in proton density
- differences of the spin-lattice or longitudinal relaxation times (T1)
- differences in the spin-spin or transverse relaxation time (T2).
When placed in a magnetic field (patient in MRI machine), gadopiclenol shortens the T1 and T2 relaxation times in targeted tissues. The extent to which a contrast agent can affect the relaxation rate of tissue water (1/T1 or 1/T2) is termed relaxivity (r1 or r2).
The relaxivity of GBCAs is presented in Table 3.Table 3. Relaxivity (r1) of GBCAs in Human Plasma/Serum at 1.5 T and 37°C Gadolinium-Chelate
r1 (L.mmol-1.s-1)
Gadobenic acid
6.3
Gadobutrol
5.2
Gadodiamide
4.3
Gadopentetic acid
4.1
Gadopiclenol
12.8
Gadoteric acid
3.6
Gadoteridol
4.1
Gadoxetic acid
6.9
Cardiac Electrophysiology
At 6 times the recommended dosage in adult patients, gadopiclenol does not prolong the QT interval to any clinically relevant extent.12.3 Pharmacokinetics
The Cmax and AUCinf of gadopiclenol increased proportionally over a dosage range from 0.025 mmol/kg to 0.3 mmol/kg (0.5 times to 6 times the recommended dosage). At the recommended dose, the mean (CV%) Cmax and AUCinf were 525 (13%) µg/mL and 569 (18%) µg·h/mL, respectively.
Distribution
After intravenous administration of ELUCIREM, gadopiclenol is distributed in the extracellular fluids.The mean (CV%) volume of distribution of gadopiclenol at steady state is 13 (13%) L.
Protein binding of gadopiclenol is ≤ 1.8% at clinically relevant concentrations.
Following GBCA administration, gadolinium is present for months or years in brain, bone, skin, and other organs [see Warnings and Precautions (5.4)]. It is unknown whether the recommended dose of ELUCIREM results in similar or different levels of gadolinium retention relative to those of other approved macrocyclic GBCAs at their recommended doses.
Elimination
The mean (CV%) elimination half-life (t1/2) of gadopiclenol is 1.5 (14%) hour.
The mean (CV%) total body clearance (CL) and renal clearance (CLr) of gadopiclenol are 100 (9.5%) mL/min and 81 (35%) mL/min, respectively.
Metabolism
Gadopiclenol is not metabolized.Excretion
Gadopiclenol is mainly eliminated through the kidneys by glomerular filtration. Approximately 98% of the dose was recovered in urine within 48 hours after administration.Specific Populations
No clinically significant differences in the pharmacokinetics of gadopiclenol were observed based on sex.
Pediatric Patients
The pharmacokinetics of gadopiclenol for pediatric patients (2 to 17 years of age) were within range to those of adults (> 18 years of age) [see Dosage and Administration (2.1)].The pharmacokinetic parameters (median [range]) of gadopiclenol in pediatric patients are presented in Table 4.
Table 4. Pharmacokinetics Parameters (Median [Range])a According to Age Classes 2-6 years
7-11 years
12-17 years
>18 years
Cl (L/h/kg)
0.12 [0.05; 0.28]
0.10 [0.04; 0.24]
0.08 [0.04; 0.20]
0.08 [0.05; 0.14]
t1/2 (h)
1.29 [0.69; 3.38]
1.48 [0.83; 3.20]
1.77 [1.00; 3.57]
1.82 [0.93; 3.68]
AUCinf (ng.h/L)
403 [169; 964]
478 [183; 1077]
582 [267; 1291]
590 [353; 937]
C20 (µg/mL)
236 [136; 387]
260 [151; 401]
286 [155; 441]
296 [166; 485]
aAt the recommended dosage
Patients with Renal Impairment
The pharmacokinetic parameters (mean (%CV)) of gadopiclenol in patients with renal impairment are presented in Table 5.
Table 5. Effect of Renal Impairment on the Pharmacokinetics of Gadopiclenola,b Normal (eGFR ≥ 90 mL/min)
Mild (eGFR 60 to < 90 mL/min)
Moderate (eGFR 30 to < 60 mL/min)
Severe (eGFR 15 to < 30 mL/min)
AUCinf (µg·h/mL)
1113 (24%)
1711 (31%)
2759 (28%)
9671 (18%)
CLr (mL/min)
96 (10%)
76 (23%)
44 (25%)
14 (26%)
t1/2 (h)
1.9
3.3
3.8
11.7
a Following administration of a single gadopiclenol 0.1 mmol/kg dose (2 times the recommended dosage).
b eGFR: estimate of GFR based on an estimation equation and expressed in mL/min. To convert mL/min/1.73 m2 to mL/min, multiply by the individual’s BSA and divide by 1.73.In patients with mild or moderate renal impairment, more than 90% of the administered ELUCIREM was recovered in urine within 48 hours. In patients with severely impaired renal function about 84% of the administered ELUCIREM was recovered in urine within 5 days.
In patients with eGFR < 15 mL/min, hemodialysis effectively removed gadopiclenol from plasma as the percentage of decrease in blood concentrations was 95 to 98% at the end of the first hemodialysis session and 100% after the third hemodialysis session [see Warnings and Precautions (5.2), Use in Specific Populations (8.6)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No carcinogenicity studies of gadopiclenol were performed.Mutagenesis
Gadopiclenol did not demonstrate mutagenic potential in in vitro bacterial reverse mutation assays (Ames test), in an in vitro chromosome aberration assay in Chinese hamster ovary cells nor in an in vivo rat micronucleus assay.Impairment of Fertility
Gadopiclenol had no effect on fertility and general reproductive performance of male and female rats when given at dose up to 10 mmol/kg (corresponding to 62 times the recommended human dose).13.2 Animal Toxicology
Local intolerance reactions, including slight to moderate erythema and edema, were observed after perivenous injection in rabbits suggesting the possibility of local irritation if the contrast medium leaks around the veins in a clinical setting [see Warnings and Precautions (5.6)].
-
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies
The safety and effectiveness of ELUCIREM for lesion visualization were evaluated in two prospective, double blind, randomized, crossover clinical studies. Study 1 (NCT03996447) was performed in adults with known or highly suspected CNS lesions with focal areas of disruption of the blood-brain barrier. Study 2 (NCT03986138) was performed in adults with suspected enhancing abnormalities in at least one body region among the head and neck, thorax, abdomen, pelvis, and musculoskeletal system.
In each study, patients received both ELUCIREM 0.05 mmol/kg and gadobutrol 0.1 mmol/kg (as an active comparator) in random order separated by 2 days to 14 days. Magnetic resonance imaging was performed before and after administration of each contrast agent.
Pre-contrast and paired (consisting of both pre-contrast and post-contrast images for the same drug) image sets were independently evaluated by three central readers who were blinded to identity of the contrast agent. Readers scored up to three lesions per patient for border delineation, internal morphology, and contrast enhancement, each on a scale from 1 to 4. The total number of lesions was also reported. An additional independent central reader performed lesion tracking to allow matching of lesions between pre-contrast and paired images.
The analysis compared the patient-level average score for matching lesions for each visualization parameter between pre-contrast and paired image sets.
14.2 Visualization of CNS Lesions
Study 1 included 256 patients with known or highly suspected CNS lesion(s). Among the enrolled patients, 239 had assessable pre-contrast and paired images with at least one matching lesion for at least one reader. These patients had a mean age of 57 years (range: 18 years to 84 years), 52% were female, and 83% were White.
All three blinded readers’ evaluations of paired pre-contrast plus post-contrast images and pre-contrast images alone for all lesion visualization criteria, the pre-specified co-primary efficacy endpoints, are presented in Table 6.
Table 6. Patient-Level CNS Lesion Visualization Scores by Reader, Paired vs. Pre-contrast in Patients Receiving ELUCIREM 0.05 mmol/kg Intravenously n
LS Mean (SE)
95% CI Difference
Paired
Pre-contrast
Difference*
Border delineation
Reader 1
227
3.90 (0.02)
2.08 (0.02)
1.82 (0.03)
(1.76, 1.88)
Reader 2
229
3.64 (0.04)
1.74 (0.04)
1.90 (0.05)
(1.81, 2.00)
Reader 3
202
3.97 (0.03)
2.61 (0.03)
1.36 (0.04)
(1.29, 1.44)
Internal morphology
Reader 1
227
3.92 (0.03)
1.66 (0.03)
2.26 (0.03)
(2.20, 2.33)
Reader 2
229
3.65 (0.03)
1.88 (0.03)
1.77 (0.04)
(1.69, 1.85)
Reader 3
202
3.97 (0.04)
2.01 (0.04)
1.96 (0.05)
(1.85, 2.06)
Degree of contrast enhancement
Reader 1
227
3.77 (0.03)
1.00 (0.03)
2.77 (0.04)
(2.69, 2.85)
Reader 2
229
3.58 (0.03)
1.00 (0.03)
2.58 (0.05)
(2.49, 2.67)
Reader 3
202
3.90 (0.02)
1.00 (0.02)
2.90 (0.03)
(2.84, 2.95)
LS: Least Squares; SE: Standard Error; CI: Confidence Interval.
Only matching lesions are considered. The mixed models based on the full analysis set (N=239) include lesion visualization factor as dependent variable, MRI modality (Precontrast and Paired MRI) as fixed factors, and patient as a random factor.
*p<0.0001 for all rowsGadopiclenol lesion visualization scores and number of lesions identified per patient were similar to those for gadobutrol.
14.3 Visualization of Body Lesions
Study 2 included 304 patients presenting with known or suspected enhancing abnormality(ies) and/or lesion(s) in at least one region among the head and neck, musculoskeletal system including extremities, and body including thorax, abdomen, and pelvis. Among the enrolled patients, 278 had assessable pre-contrast and paired images with at least one matching lesion for at least one reader. These patients had a mean age of 57 years (range: 21 years to 86 years), 59% were female, and 71% were White.
Three readers assessed images of the head and neck, three other readers assessed images of the musculoskeletal system, and another three readers assessed other areas collectively referred to as the body (thorax, abdomen, and pelvis). Lesion visualization scores by reader in each anatomic region at patient-level as supportive analyses are summarized in Table 7.
Table 7. Patient-Level Body Lesion Visualization Scores by Reader and Anatomic Region, Paired vs. Pre-contrast in Patients Receiving ELUCIREM 0.05 mmol/kg Intravenously
n
LS Mean (SE)
95% CI
difference
Paired
Pre-contrast
Difference
Head & Neck
Border delineation
Reader 1
15
3.71 (0.10)
2.13 (0.10)
1.58 (0.14)
(1.30, 1.86)
Reader 2
19
3.53 (0.18)
2.11 (0.18)
1.42 (0.18)
(1.06, 1.78)
Reader 3
13
3.92 (0.13)
2.85 (0.13)
1.08 (0.13)
(0.82, 1.33)
Internal morphology
Reader 1
15
3.80 (0.07)
1.87 (0.07)
1.93 (0.10)
(1.74, 2.12)
Reader 2
19
3.74 (0.14)
2.05 (0.14)
1.68 (0.16)
(1.37, 2.00)
Reader 3
13
3.92 (0.12)
2.54 (0.12)
1.38 (0.14)
(1.10, 1.67)
Degree of contrast enhancement
Reader 1
15
3.60 (0.11)
1.00 (0.11)
2.60 (0.16)
(2.29, 2.91)
Reader 2
19
3.68 (0.16)
1.00 (0.16)
2.68 (0.22)
(2.22, 3.15)
Reader 3
13
3.92 (0.11)
1.00 (0.11)
2.92 (0.15)
(2.61, 3.24)
Musculoskeletal system (including extremities)
Border delineation
Reader 1
17
3.00 (0.10)
2.06 (0.10)
0.94 (0.13)
(0.68, 1.20)
Reader 2
17
2.68 (0.20)
2.44 (0.20)
0.24 (0.19)
(-0.15, 0.62)
Reader 3
21
2.81 (0.10)
2.05 (0.10)
0.76 (0.10)
(0.56, 0.96)
Internal morphology
Reader 1
17
3.00 (0.07)
2.00 (0.07)
1.00 (0.09)
(0.82, 1.18)
Reader 2
17
3.94 (0.15)
2.35 (0.15)
1.59 (0.17)
(1.25, 1.92)
Reader 3
21
2.90 (0.09)
2.05 (0.09)
0.86 (0.11)
(0.64, 1.08)
Degree of contrast enhancement
Reader 1
17
2.82 (0.10)
1.00 (0.10)
1.82 (0.15)
(1.53, 2.12)
Reader 2
17
3.33 (0.17)
1.00 (0.17)
2.33 (0.24)
(1.847, 2.82)
Reader 3
21
3.06 (0.08)
1.00 (0.08)
2.06 (0.12)
(1.82, 2.31)
Body (thorax, abdomen, pelvis)
Border delineation
Reader 1
219
3.86 (0.03)
2.28 (0.03)
1.57 (0.04)
(1.50, 1.64)
Reader 2
194
3.54 (0.06)
3.15 (0.06)
0.40 (0.06)
(0.29, 0.51)
Reader 3
228
3.53 (0.03)
1.69 (0.03)
1.84 (0.03)
(1.78, 1.90)
Internal morphology
Reader 1
219
3.86 (0.02)
2.00 (0.02)
1.87 (0.03)
(1.82, 1.92)
Reader 2
194
3.74 (0.05)
3.41 (0.05)
0.33 (0.05)
(0.23, 0.43)
Reader 3
228
3.78 (0.03)
1.60 (0.03)
2.17 (0.03)
(2.11, 2.24)
Degree of contrast enhancement
Reader 1
219
3.71 (0.03)
1.00 (0.03)
2.71 (0.04)
(2.63, 2.79)
Reader 2
194
2.69 (0.05)
1.00 (0.05)
1.69 (0.07)
(1.54, 1.83)
Reader 3
228
3.33 (0.03)
1.00 (0.03)
2.33 (0.44)
(2.25, 2.40)
LS: Least Squares; SE: Standard Error; CI: Confidence Interval.
Only matching lesions are considered. The mixed models based on the full analysis set (N=278) include lesion visualization factor as a dependent variable, patient as a random factor, and MRI modality (Pre-contrast and Paired MRI), body regions, and MRI body regions as fixed factors.Gadopiclenol lesion visualization scores and number of lesions identified per patient were similar to those for gadobutrol.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
HOW SUPPLIED
ELUCIREM is a clear, colorless to yellow aqueous solution supplied in the following presentations:Strength
Sale Unit
NDC
Single-Dose Vial (glass)
1.5 mmol/3 mL (0.5 mmol/mL)
Carton of 1
67684-4230-1
Carton of 10
67684-4230-2
3.75 mmol/7.5 mL (0.5 mmol/mL)
Carton of 1
67684-4231-1 Carton of 10
67684-4231-2
5 mmol/10 mL (0.5 mmol/mL)
Carton of 1
67684-4232-1
Carton of 10
67684-4232-2
7.5 mmol/15 mL (0.5 mmol/mL)
Carton of 1
67684-4233-1
Carton of 10
67684-4233-2
Single-Dose Prefilled Syringe (plastic)
3.75 mmol/7.5 mL (0.5 mmol/mL)
Carton of 1
67684-4240-1
Carton of 10
67684-4240-2
5 mmol/10 mL (0.5 mmol/mL)
Carton of 1
67684-4241-1
Carton of 10
67684-4241-2
7.5 mmol/15 mL (0.5 mmol/mL)
Carton of 1
67684-4242-1
Carton of 10
67684-4242-2
Pharmacy Bulk Package (glass)
15 mmol/30 mL (0.5 mmol/mL)
Carton of 1
67684-4250-1
Carton of 25
67684-4250-2
25 mmol/50 mL (0.5 mmol/mL)
Carton of 1
67684-4250-3
Carton of 25
67684-4250-4
50 mmol/100 mL (0.5 mmol/mL)
Carton of 1
67684-4250-5
Carton of 6
67684-4250-6
Carton of 12
67684-4250-7
Imaging Bulk Package (glass)
15 mmol/30 mL (0.5 mmol/mL)
Carton of 1
67684-4251-1
Carton of 25
67684-4251-2
25 mmol/50 mL (0.5 mmol/mL)
Carton of 1
67684-4251-3
Carton of 25
67684-4251-4
50 mmol/100 mL (0.5 mmol/mL)
Carton of 1
67684-4251-5
Carton of 6
67684-4251-6
Carton of 12
67684-4251-7
Storage and Handling
Store at 25°C (77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP, Controlled Room Temperature].Do not freeze Pre-filled syringes.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Nephrogenic Systemic Fibrosis
Inform the patient that ELUCIREM may increase the risk for NSF among patients with impaired elimination of the drugs and that NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs.
Instruct the patients to contact their physician if they develop signs or symptoms of NSF following ELUCIREM administration, such as burning, itching, swelling, scaling, hardening and tightening of the skin; red or dark patches on the skin; stiffness in joints with trouble moving, bending or straightening the arms, hands, legs or feet; pain in the hip bones or ribs; or muscle weakness [see Warnings and Precautions (5.2)].
Gadolinium Retention
Advise patients that gadolinium is retained for months or years in brain, bone, skin, and other organs following ELUCIREM administration even in patients with normal renal function. The clinical consequences of retention are unknown. Retention depends on multiple factors and is greater following administration of linear GBCAs than following administration of macrocyclic GBCAs [see Warnings and Precautions (5.4)].
Injection Site Reactions
Inform the patient that ELUCIREM may cause reactions along the venous injection site, such as mild and transient burning or pain or feeling of warmth or coldness at the injection site [see Warnings and Precautions (5.6)].
Pregnancy
Advise pregnant women of the potential risk of fetal exposure to ELUCIREM [see Use in Specific Populations (8.1)].
Vials and pre-filled syringes manufactured by Liebel-Flarsheim Company LLC,
8800 Durant Road, Raleigh, North Carolina (NC) 27616-3104, USAVials manufactured by BIPSO GmbH
Robert-Gerwig-Strasse 4, Singen (Hohentwiel) 78224, GermanyDistributed by
Guerbet LLC
214 Carnegie Center, Suite 300, Princeton, NJ 08540, USA -
MEDICATION GUIDE
MEDICATION GUIDE
ELUCIREMTM (ah LOOS er em)
(gadopiclenol)
injection, for intravenous useWhat is the most important information I should know about ELUCIREM?
- GBCAs like ELUCIREM may cause serious side effects including death, coma, encephalopathy, and seizures when it is given intrathecally (injection given into the spinal canal). It is not known if ELUCIREM is safe and effective with intrathecal use. ELUCIREM is not approved for this use.
- ELUCIREM contains a metal called gadolinium. Small amounts of gadolinium can stay in your body including the brain, bones, skin and other parts of your body for a long time (several months to years).
- It is not known how gadolinium may affect you, but so far, studies have not found harmful effects in patients with normal kidneys.
- Rarely patients have reported pains, tiredness, and skin, muscle or bone ailments for a long time, but these symptoms have not been directly linked to gadolinium.
- There are different GBCAs that can be used for your MRI exam. The amount of gadolinium that stays in the body is different for different gadolinium medicines. Gadolinium stays in the body more after gadodiamide than after gadoxetate disodium or gadobenate dimeglumine. Gadolinium stays in the body the least after, gadoterate meglumine, gadobutrol, gadoteridol, and gadopiclenol.
- People who get many doses of gadolinium medicines, women who are pregnant and young children may be at increased risk from gadolinium staying in the body.
- Some people with kidney problems who get gadolinium medicines can develop a condition with severe thickening of the skin, muscles and other organs in the body (nephrogenic systemic fibrosis). Your healthcare provider should screen you to see how well your kidneys are working before you receive ELUCIREM.
What is ELUCIREM?
- ELUCIREM is a prescription medicine called a gadolinium-based contrast agent (GBCA). ELUCIREM, like other GBCAs, is injected into your vein and used with a magnetic resonance imaging (MRI) scanner.
- An MRI exam with a GBCA, including ELUCIREM, helps your healthcare provider to see problems better than an MRI exam without a GBCA.
- Your healthcare provider has reviewed your medical records and has determined that you would benefit from using a GBCA with your MRI exam.
Do not receive ELUCIREM if you have had a severe allergic reaction to ELUCIREM.
Before receiving ELUCIREM, tell your healthcare provider about all your medical conditions, including if you:
- have had any MRI procedures in the past where you received a GBCA. Your healthcare provider may ask you for more information including the dates of these MRI procedures.
- are pregnant or plan to become pregnant. It is not known if ELUCIREM can harm your unborn baby. Talk to your healthcare provider about the possible risks to an unborn baby if a GBCA such as ELUCIREM is received during pregnancy.
- have kidney problems, diabetes, or high blood pressure.
- have had an allergic reaction to dyes (contrast agents) including GBCAs.
What are possible side effects of ELUCIREM?
- See “What is the most important information I should know about ELUCIREM?”
- Allergic reactions. ELUCIREM can cause allergic reactions that can sometimes be serious. Your healthcare provider will monitor you closely for symptoms of an allergic reaction.
The most common side effects of ELUCIREM include: injection site pain, headache, nausea, injection site coldness, injection site warmth, dizziness, and localized swelling.
These are not all the possible side effects of ELUCIREM.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective uses of ELUCIREM.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your healthcare provider for information about ELUCIREM that is written for health professionals.What are the ingredients in ELUCIREM?
Active ingredient: gadopiclenol
Inactive ingredients: tetraxetan; trometamol; hydrochloric acid or sodium hydroxide for pH adjustment; and water for injection
Manufactured by: Vials and pre-filled syringes manufactured by Liebel-Flarsheim Company LLC, 8800 Durant Road, Raleigh, North Carolina (NC) 27616-3104, USA
Vials manufactured by BIPSO GmbH Robert-Gerwig-Strasse 4, Singen (Hohentwiel) 78224, Germany
Distributed by: Guerbet LLC, 214 Carnegie Center, Suite 300, Princeton, New Jersey 08540, USA
For more information, go to www.guerbet-us.com or call 1-877-729-6679.This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 12/2025
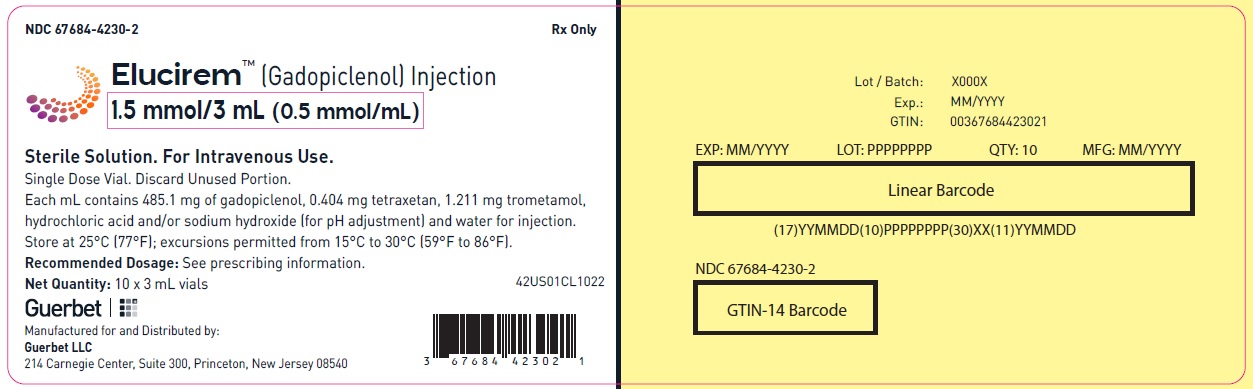
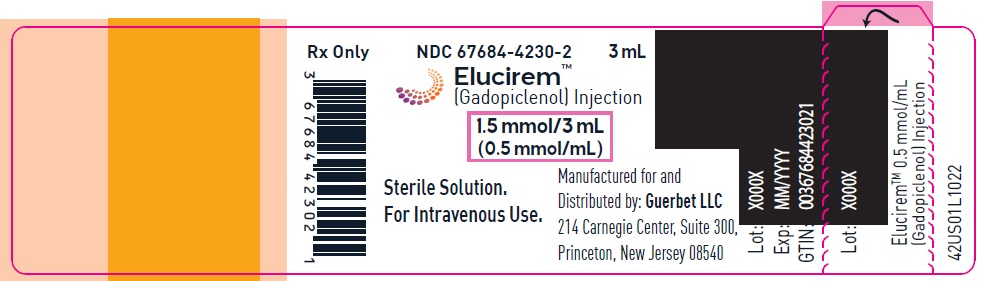
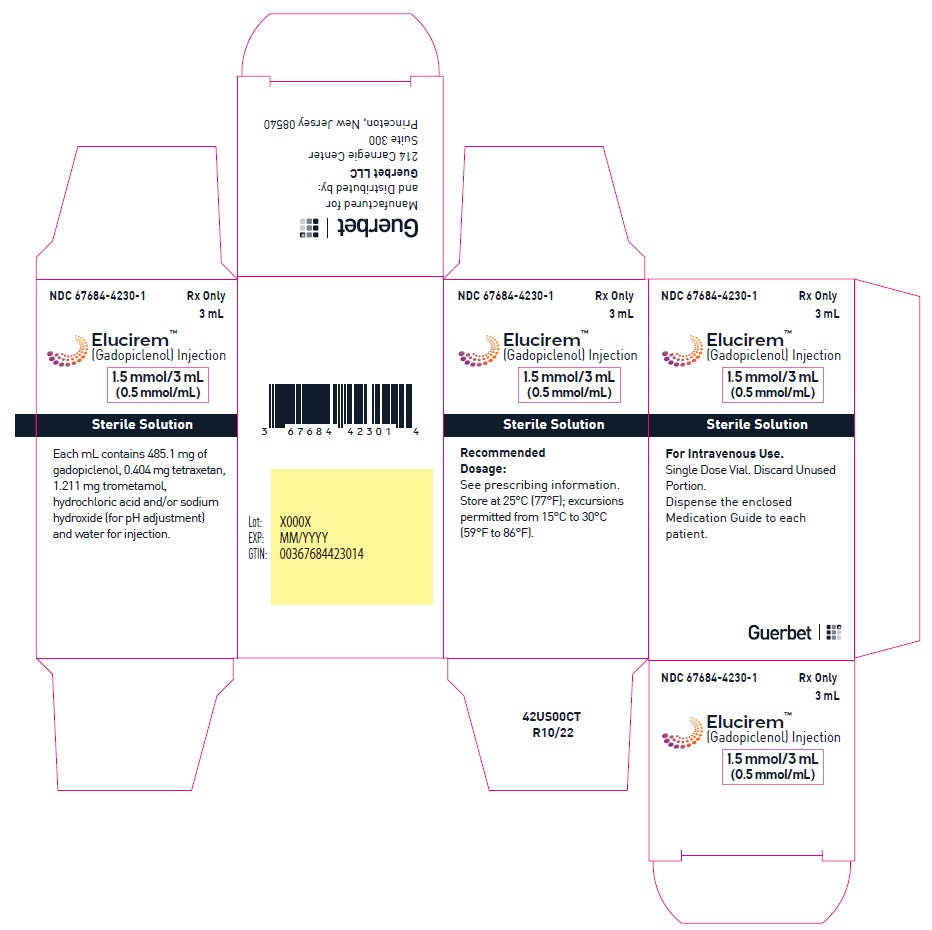
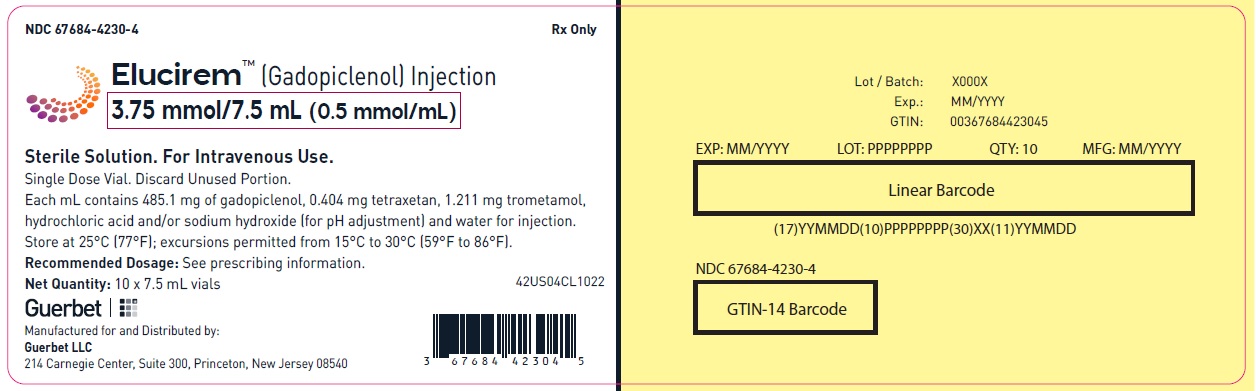
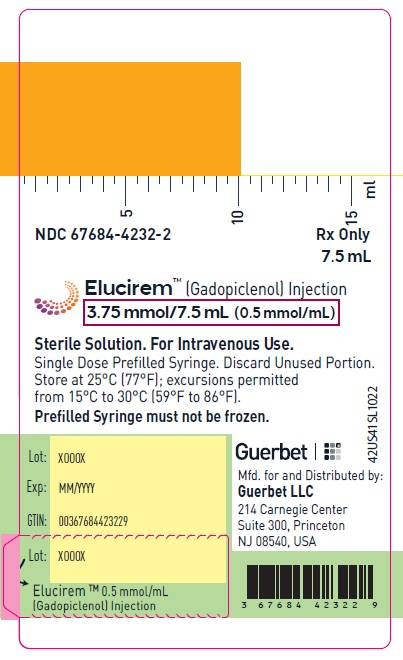
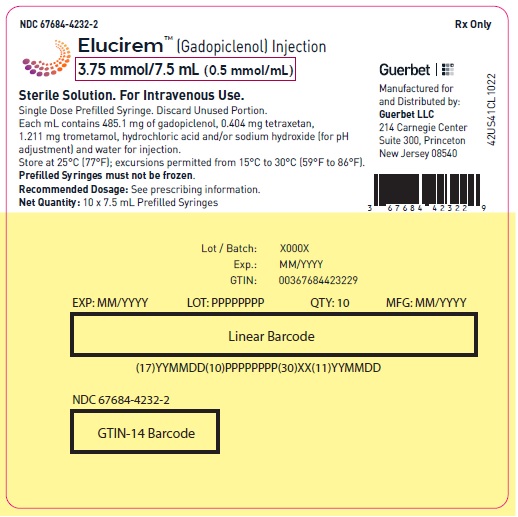
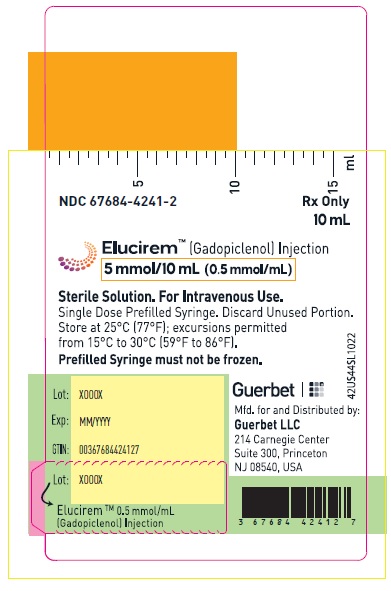
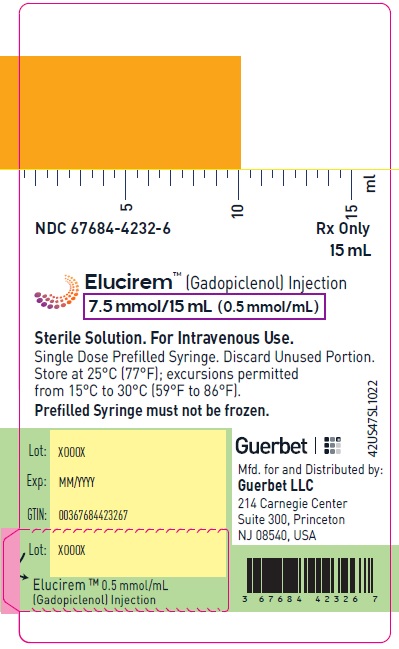
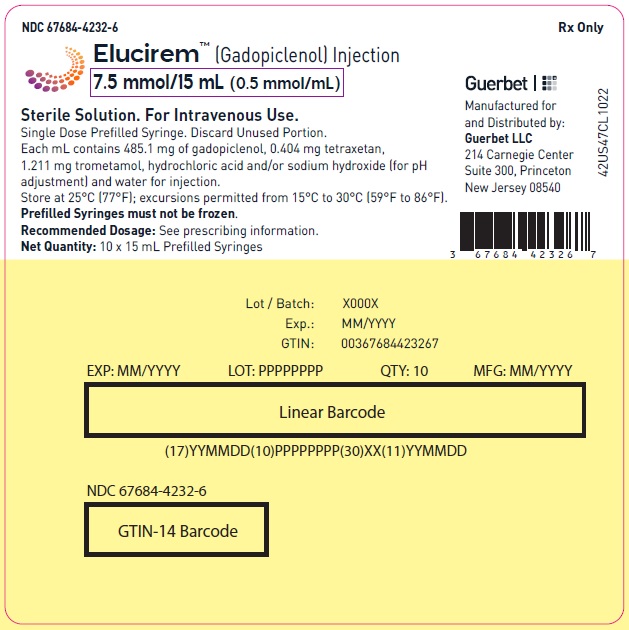
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ELUCIREM
gadopiclenol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67684-4230 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Gadopiclenol (UNII: S276568KOY) (Gadopiclenol - UNII:S276568KOY) Gadopiclenol 485.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Tetraxetan (UNII: 1HTE449DGZ) Sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Tromethamine (UNII: 023C2WHX2V) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67684-4230-1 1 in 1 CARTON 09/21/2022 1 3 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 67684-4230-2 10 in 1 CARTON 09/21/2022 2 3 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216986 09/21/2022 ELUCIREM
gadopiclenol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67684-4231 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Gadopiclenol (UNII: S276568KOY) (Gadopiclenol - UNII:S276568KOY) Gadopiclenol 485.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Tetraxetan (UNII: 1HTE449DGZ) Sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Tromethamine (UNII: 023C2WHX2V) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67684-4231-1 1 in 1 CARTON 09/21/2022 1 7.5 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 67684-4231-2 10 in 1 CARTON 09/21/2022 2 7.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216986 09/21/2022 ELUCIREM
gadopiclenol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67684-4232 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Gadopiclenol (UNII: S276568KOY) (Gadopiclenol - UNII:S276568KOY) Gadopiclenol 485.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Tetraxetan (UNII: 1HTE449DGZ) Sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Tromethamine (UNII: 023C2WHX2V) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67684-4232-1 1 in 1 CARTON 09/21/2022 1 10 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 67684-4232-2 10 in 1 CARTON 09/21/2022 2 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216986 09/21/2022 ELUCIREM
gadopiclenol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67684-4233 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Gadopiclenol (UNII: S276568KOY) (Gadopiclenol - UNII:S276568KOY) Gadopiclenol 485.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Tetraxetan (UNII: 1HTE449DGZ) Sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Tromethamine (UNII: 023C2WHX2V) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67684-4233-1 1 in 1 CARTON 09/21/2022 1 15 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 67684-4233-2 10 in 1 CARTON 09/21/2022 2 15 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216986 09/21/2022 ELUCIREM
gadopiclenol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67684-4240 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Gadopiclenol (UNII: S276568KOY) (Gadopiclenol - UNII:S276568KOY) Gadopiclenol 485.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Tetraxetan (UNII: 1HTE449DGZ) Sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Tromethamine (UNII: 023C2WHX2V) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67684-4240-1 1 in 1 CARTON 09/21/2022 1 7.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 67684-4240-2 10 in 1 CARTON 09/21/2022 2 7.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216986 09/21/2022 ELUCIREM
gadopiclenol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67684-4241 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Gadopiclenol (UNII: S276568KOY) (Gadopiclenol - UNII:S276568KOY) Gadopiclenol 485.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Tetraxetan (UNII: 1HTE449DGZ) Sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Tromethamine (UNII: 023C2WHX2V) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67684-4241-1 1 in 1 CARTON 09/21/2022 1 10 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 67684-4241-2 10 in 1 CARTON 09/21/2022 2 10 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216986 09/21/2022 ELUCIREM
gadopiclenol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67684-4242 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Gadopiclenol (UNII: S276568KOY) (Gadopiclenol - UNII:S276568KOY) Gadopiclenol 485.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Tetraxetan (UNII: 1HTE449DGZ) Sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Tromethamine (UNII: 023C2WHX2V) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67684-4242-1 1 in 1 CARTON 09/21/2022 1 15 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 67684-4242-2 10 in 1 CARTON 09/21/2022 2 15 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216986 09/21/2022 ELUCIREM
gadopiclenol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67684-4250 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Gadopiclenol (UNII: S276568KOY) (Gadopiclenol - UNII:S276568KOY) Gadopiclenol 485.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Tetraxetan (UNII: 1HTE449DGZ) Sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Tromethamine (UNII: 023C2WHX2V) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67684-4250-1 1 in 1 CARTON 09/21/2022 1 30 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 2 NDC: 67684-4250-2 25 in 1 CARTON 09/21/2022 2 30 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 3 NDC: 67684-4250-3 1 in 1 CARTON 09/21/2022 3 50 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 4 NDC: 67684-4250-4 25 in 1 CARTON 09/21/2022 4 50 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 5 NDC: 67684-4250-5 1 in 1 CARTON 09/21/2022 5 100 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 6 NDC: 67684-4250-6 6 in 1 CARTON 09/21/2022 6 100 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 7 NDC: 67684-4250-7 12 in 1 CARTON 09/21/2022 7 100 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216986 09/21/2022 ELUCIREM
gadopiclenol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67684-4251 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Gadopiclenol (UNII: S276568KOY) (Gadopiclenol - UNII:S276568KOY) Gadopiclenol 485.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength Tetraxetan (UNII: 1HTE449DGZ) Sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Tromethamine (UNII: 023C2WHX2V) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67684-4251-1 1 in 1 CARTON 11/14/2025 1 30 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 2 NDC: 67684-4251-2 25 in 1 CARTON 11/14/2025 2 30 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 3 NDC: 67684-4251-3 1 in 1 CARTON 11/14/2025 3 50 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 4 NDC: 67684-4251-4 25 in 1 CARTON 11/14/2025 4 50 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 5 NDC: 67684-4251-5 1 in 1 CARTON 11/14/2025 5 100 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 6 NDC: 67684-4251-6 6 in 1 CARTON 11/14/2025 6 100 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product 7 NDC: 67684-4251-7 12 in 1 CARTON 11/14/2025 7 100 mL in 1 VIAL, PHARMACY BULK PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216986 11/14/2025 Labeler - Guerbet LLC (037876096) Establishment Name Address ID/FEI Business Operations Liebel-Flarsheim Company LLC 109024984 manufacture(67684-4230, 67684-4232, 67684-4231, 67684-4233, 67684-4240, 67684-4241, 67684-4242, 67684-4250, 67684-4251) , pack(67684-4230, 67684-4231, 67684-4232, 67684-4233, 67684-4240, 67684-4241, 67684-4242, 67684-4250, 67684-4251) , label(67684-4230, 67684-4231, 67684-4232, 67684-4233, 67684-4240, 67684-4241, 67684-4242, 67684-4250, 67684-4251) Establishment Name Address ID/FEI Business Operations Guerbet 503171949 api manufacture(67684-4230, 67684-4231, 67684-4232, 67684-4233, 67684-4240, 67684-4241, 67684-4242, 67684-4250, 67684-4251) Establishment Name Address ID/FEI Business Operations BIPSO GmbH 342104149 manufacture(67684-4230, 67684-4231, 67684-4232, 67684-4233, 67684-4250, 67684-4251)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.