FOSFOMYCIN TROMETHAMINE powder
Fosfomycin Tromethamine by
Drug Labeling and Warnings
Fosfomycin Tromethamine by is a Prescription medication manufactured, distributed, or labeled by Zambon USA, Ltd, Zambon Switzerland Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
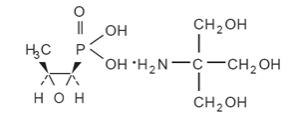
Fosfomycin tromethamine granules for oral solution contains fosfomycin tromethamine, a synthetic, broad spectrum, bactericidal antibiotic for oral administration. It is available as a single-dose sachet which contains white granules consisting of 5.631 grams of fosfomycin tromethamine (equivalent to 3 grams of fosfomycin), and the following inactive ingredients: mandarin flavor, orange flavor, saccharin, and sucrose. The contents of the sachet must be dissolved in water. Fosfomycin tromethamine, a phosphonic acid derivative, is available as (1 R,2 S)-(1,2-epoxypropyl)phosphonic acid, compound with 2-amino-2-(hydroxymethyl)-1,3-propanediol (1:1). It is a white granular compound with a molecular weight of 259.2. Its empirical formula is C 3H 7O 4PC 4H 11NO 3, and its chemical structure is as follows:
-
CLINICAL PHARMACOLOGY
Absorption: Fosfomycin tromethamine is rapidly absorbed following oral administration and converted to the free acid, fosfomycin. Absolute oral bioavailability under fasting conditions is 37%. After a single 3-gram dose of fosfomycin tromethamine, the mean (± 1 SD) maximum serum concentration (C max) achieved was 26.1 (± 9.1) mcg/mL within 2 hours. The oral bioavailability of fosfomycin is reduced to 30% under fed conditions. Following a single 3-gram oral dose of fosfomycin tromethamine with a high-fat meal, the mean C max achieved was 17.6 (± 4.4) mcg/mL within 4 hours.
Cimetidine does not affect the pharmacokinetics of fosfomycin when coadministered with fosfomycin tromethamine. Metoclopramide lowers the serum concentrations and urinary excretion of fosfomycin when coadministered with fosfomycin tromethamine. (See PRECAUTIONS, Drug Interactions.)
Distribution: The mean apparent steady-state volume of distribution (V ss) is 136.1 (±44.1) L following oral administration of fosfomycin tromethamine. Fosfomycin is not bound to plasma proteins.
Fosfomycin is distributed to the kidneys, bladder wall, prostate, and seminal vesicles. Following a 50 mg/kg dose of fosfomycin to patients undergoing urological surgery for bladder carcinoma, the mean concentration of fosfomycin in the bladder, taken at a distance from the neoplastic site, was 18.0 mcg per gram of tissue at 3 hours after dosing. Fosfomycin has been shown to cross the placental barrier in animals and man.
Excretion: Fosfomycin is excreted unchanged in both urine and feces. Following oral administration of fosfomycin tromethamine, the mean total body clearance (CL TB) and mean renal clearance (CL R) of fosfomycin were 16.9 (± 3.5) L/hr and 6.3 (± 1.7) L/hr, respectively. Approximately 38% of a 3-gram dose of fosfomycin tromethamine is recovered from urine, and 18% is recovered from feces. Following intravenous administration, the mean CL TB and mean CL R of fosfomycin were 6.1 (±1.0) L/hr and 5.5 (± 1.2) L/hr, respectively.
A mean urine fosfomycin concentration of 706 (± 466) mcg/mL was attained within 2-4 hours after a single oral 3-gm dose of fosfomycin tromethamine under fasting conditions. The mean urinary concentration of fosfomycin was 10 mcg/mL in samples collected 72-84 hours following a single oral dose of fosfomycin tromethamine.
Following a 3-gram dose of fosfomycin tromethamine administered with a high fat meal, a mean urine fosfomycin concentration of 537 (± 252) mcg/mL was attained within 6-8 hours. Although the rate of urinary excretion of fosfomycin was reduced under fed conditions, the cumulative amount of fosfomycin excreted in the urine was the same, 1118 (± 201) mg (fed) vs. 1140 mg (± 238) (fasting). Further, urinary concentrations equal to or greater than 100 mcg/mL were maintained for the same duration, 26 hours, indicating that fosfomycin tromethamine can be taken without regard to food.
Following oral administration of fosfomycin tromethamine, the mean half-life for elimination (t 1/2) is 5.7 (± 2.8) hours.
Special Populations:
Geriatric: Based on limited data regarding 24-hour urinary drug concentrations, no differences in urinary excretion of fosfomycin have been observed in elderly subjects. No dosage adjustment is necessary in the elderly.
Gender: There are no gender differences in the pharmacokinetics of fosfomycin.
Renal Insufficiency: In 5 anuric patients undergoing hemodialysis, the t 1/2 of fosfomycin during hemodialysis was 40 hours. In patients with varying degrees of renal impairment (creatinine clearances varying from 54 mL/min to 7 mL/min), the t 1/2 of fosfomycin increased from 11 hours to 50 hours. The percent of fosfomycin recovered in urine decreased from 32% to 11% indicating that renal impairment significantly decreases the excretion of fosfomycin.
Microbiology
Fosfomycin (the active component of fosfomycin tromethamine) has in vitro activity against a broad range of gram-positive and gram-negative aerobic microorganisms which are associated with uncomplicated urinary tract infections. Fosfomycin is bactericidal in urine at therapeutic doses. The bactericidal action of fosfomycin is due to its inactivation of the enzyme enolpyruvyl transferase, thereby irreversibly blocking the condensation of uridine diphosphate-N-acetylglucosamine with p-enolpyruvate, one of the first steps in bacterial cell wall synthesis. It also reduces adherence of bacteria to uroepithelial cells.
There is generally no cross-resistance between fosfomycin and other classes of antibacterial agents such as beta-lactams and aminoglycosides.
Fosfomycin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
Aerobic gram-positive microorganisms
Enterococcus faecalisAerobic gram-negative microorganisms
Escherichia coliThe following in vitro data are available, but theirclinical significance is unknown.
Fosfomycin exhibits in vitro minimum inhibitory concentrations (MIC’s) of 64 mcg/mL or less against most (≥ 90%) strains of the following microorganisms; however, the safety and effectiveness of fosfomycin in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled clinical trials:
Aerobic gram-positive microorganisms
Enterococcus faeciumAerobic gram-negative microorganisms
Citrobacter diversus
Citrobacter freundii
Enterobacter aerogenes
Klebsiella oxytoca
Klebsiella pneuomoniae
Proteus mirabilis
Proteus vulgaris
Serratia marcescensSUSCEPTIBILITY TESTING
Dilution Techniques:
Quantitative methods are used to determine minimum inhibitory concentrations (MIC’s). These MIC’s provide estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure uses a standardized agar dilution method 1 or equivalent with standardized inoculum concentrations and standardized concentrations of fosfomycin tromethamine (in terms of fosfomycin base content) powder supplemented with 25 mcg/mL of glucose-6-phosphate. BROTH DILUTION METHODS SHOULDNOT BE USED TO TEST SUSCEPTIBILITY TO FOSFOMYCIN. The MIC values obtained should be interpreted according to the following criteria:
MIC (mcg/mL) Interpretation ≤ 64 Susceptible (S) 128 Intermediate (I) ≥ 256 Resistant (R) A report of “susceptible” indicates that the pathogen is likely to be inhibited by usually achievable concentrations of the antimicrobial compound in the urine. A report of “intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “resistant” indicates that usually achievable concentrations of the antimicrobial compound in the urine are unlikely to be inhibitory and that other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms. Standard fosfomycin tromethamine powder should provide the following MIC values for agar dilution testing in media containing 25 mcg/mL of glucose-6-phosphate. [Broth dilution testing should not be performed].
Microorganism MIC (mcg/mL) Enterococcus faecalis ATCC 29212 32-128 Escherichia coli ATCC 25922 0.5-2 Pseudomonas aeruginosa ATCC 27853 2-8 Staphylococcus aureus ATCC 29213 0.5-4 Diffusion Techniques:
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial agents. One such standardized procedure 2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 200-mcg fosfomycin and 50-mcg of glucose-6-phosphate to test the susceptibility of microorganisms to fosfomycin.
Reports from the laboratory providing results of the standard single-disk susceptibility tests with disks containing 200-mcg of fosfomycin and 50-mcg of glucose-6-phosphate should be interpreted according to the following criteria:
Zone Diameter (mm) Interpretation ≥ 16 Susceptible (S) 13-15 Intermediate (I) ≤ 12 Resistant (R) Interpretation should be stated as above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for fosfomycin.
As with standardized dilution techniques, diffusion methods require use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 200-mcg fosfomycin disk with the 50-mcg of glucose-6-phosphate should provide the following zone diameters in these laboratory quality control strains:
Microorganism Zone Diameter (mm) Escherichia coli ATCC 25922 22-30 Staphylococcus aureus ATCC 25923 25-33 -
INDICATIONS AND USAGE
Fosfomycin tromethamine is indicated only for the treatment of uncomplicated urinary tract infections (acute cystitis) in women due to susceptible strains of Escherichia coli and Enterococcus faecalis. Fosfomycin tromethamine is not indicated for the treatment of pyelonephritis or perinephric abscess.
If persistence or reappearance of bacteriuria occurs after treatment with fosfomycin tromethamine, other therapeutic agents should be selected. (See PRECAUTIONS and CLINICAL STUDIES sections.)
- CONTRAINDICATIONS
-
WARNINGS
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including fosfomycin tromethamine, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C.difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
Do not use more than one single dose of fosfomycin tromethamine to treat a single episode of acute cystitis. Repeated daily doses of fosfomycin tromethamine did not improve the clinical success or microbiological eradication rates compared to single dose therapy, but did increase the incidence of adverse events. Urine specimens for culture and susceptibility testing should be obtained before and after completion of therapy.
Information for Patients
Patients should be informed:
- That fosfomycin tromethamine can be taken with or without food.
- That their symptoms should improve in two to three days after taking fosfomycin tromethamine; if not improved, the patient should contact her health care provider.
- Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug Interactions
Metoclopramide: When coadministered with fosfomycin tromethamine, metoclopramide, a drug which increases gastrointestinal motility, lowers the serum concentration and urinary excretion of fosfomycin. Other drugs that increase gastrointestinal motility may produce similar effects.
Cimetidine: Cimetidine does not affect the pharmacokinetics of fosfomycin when coadministered with fosfomycin tromethamine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term carcinogenicity studies in rodents have not been conducted because fosfomycin tromethamine is intended for single dose treatment in humans. Fosfomycin tromethamine was not mutagenic or genotoxic in the in vitro Ames’ bacterial reversion test, in cultured human lymphocytes, in Chinese hamster V79 cells, and the in vivo mouse micronucleus assay. Fosfomycin tromethamine did not affect fertility or reproductive performance in male and female rats.
Pregnancy:
Teratogenic Effects
When administered intramuscularly as the sodium salt at a dose of 1 gram to pregnant women, fosfomycin crosses the placental barrier. Fosfomycin tromethamine crosses the placental barrier of rats; it does not produce teratogenic effects in pregnant rats at dosages as high as 1000 mg/kg/day (approximately 9 and 1.4 times the human dose based on body weight and mg/m 2, respectively). When administered to pregnant female rabbits at dosages as high as 1000 mg/kg/day (approximately 9 and 2.7 times the human dose based on body weight and mg/m 2, respectively), fetotoxicities were observed. However, these toxicities were seen at maternally toxic doses and were considered to be due to the sensitivity of the rabbit to changes in the intestinal microflora resulting from the antibiotic administration. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
It is not known whether fosfomycin tromethamine is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from fosfomycin tromethamine, a decision should be made whether to discontinue nursing or to not administer the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in children age 12 years and under have not been established in adequate and well-controlled studies.
Geriatric Use
Clinical studies of fosfomycin tromethamine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Clinical Trials:
In clinical studies, drug related adverse events which were reported in greater than 1% of the fosfomycin-treated study population are listed below:
Drug-Related Adverse Events (%) in Fosfomycin and Comparator Populations Adverse
EventsFosfomycin
N=1233Nitrofurantoin
N=374Trimethoprim/
sulfamethoxazole
N=428Ciprofoxacin
N=455Diarrhea 9.0 6.4 2.3 3.1 Vaginitis 5.5 5.3 4.7 6.3 Nausea 4.1 7.2 8.6 3.4 Headache 3.9 5.9 5.4 3.4 Dizziness 1.3 1.9 2.3 2.2 Asthenia 1.1 0.3 0.5 0.0 Dyspepsia 1.1 2.1 0.7 1.1 In clinical trials, the most frequently reported adverse events occurring in > 1% of the study population regardless of drug relationship were: diarrhea 10.4%, headache 10.3%, vaginitis 7.6%, nausea 5.2%, rhinitis 4.5%, back pain 3.0%, dysmenorrheal 2.6%, pharyngitis 2.5%, dizziness 2.3%, abdominal pain 2.2%, pain 2.2%, dyspepsia 1.8%, asthenia 1.7%, and rash 1.4%.
The following adverse events occurred in clinical trials at a rate of less than 1%, regardless of drug relationship: abnormal stools, anorexia, constipation, dry mouth, dysuria, ear disorder, fever, flatulence, flu syndrome, hematuria, infection, insomnia, lymphadenopathy, menstrual disorder, migraine, myalgia, nervousness, paresthesia, pruritus, SGPT increased, skin disorder, somnolence, and vomiting.
One patient developed unilateral optic neuritis, an event considered possibly related to fosfomycin tromethamine therapy.
Post-marketing Experience:
Serious adverse events from the marketing experience with fosfomycin tromethamine outside of the United States have been rarely reported and include: angioedema, aplastic anemia, asthma (exacerbation), cholestatic jaundice, hepatic necrosis, and toxic megacolon.
Although causality has not been established, during post marketing surveillance, the following events have occurred in patients prescribed fosfomycin tromethamine: anaphylaxis and hearing loss.
Laboratory Changes:
Significant laboratory changes reported in U.S. clinical trials of fosfomycin tromethamine without regard to drug relationship include: increased eosinophil count, increased or decreased WBC count, increased bilirubin, increased SGPT, increased SGOT, increased alkaline phosphatase, decreased hematocrit, decreased hemoglobin, increased and decreased platelet count. The changes were generally transient and were not clinically significant.
-
OVERDOSAGE
In acute toxicology studies, oral administration of high doses of fosfomycin tromethamine up to 5 g/kg were well-tolerated in mice and rats, produced transient and minor incidences of watery stools in rabbits, and produced diarrhea with anorexia in dogs occurring 2-3 days after single dose administration. These doses represent 50-125 times the human therapeutic dose.
The following events have been observed in patients who have taken fosfomycin tromethamine in overdose: vestibular loss, impaired hearing, metallic taste, and general decline in taste perception. In the event of overdosage, treatment should be symptomatic and supportive.
-
DOSAGE AND ADMINISTRATION
The recommended dosage for women 18 years of age and older for uncomplicated urinary tract infection (acute cystitis) is one sachet of fosfomycin tromethamine. Fosfomycin tromethamine may be taken with or without food.
Fosfomycin tromethamine should not be taken in its dry form. Always mix fosfomycin tromethamine with water before ingesting. (See PREPARATION section.)
- PREPARATION
-
HOW SUPPLIED
Fosfomycin tromethamine granules for oral solution is available as a single-dose sachet containing the equivalent of 3 grams of fosfomycin.
NDC
Single-dose sachet 82036-4274-8
One unit carton 82036-4274-1
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
Keep this and all drugs out of the reach of children.
-
CLINICAL STUDIES
In controlled, double-blind studies of acute cystitis performed in the United States, a single-dose of fosfomycin tromethamine was compared to three other oral antibiotics (See table below). The study population consisted of patients with symptoms and signs of acute
cystitis of less than 4 days duration, no manifestations of upper tract infection (e.g., flank pain, chills, fever), no history of recurrent urinary tract infections (20% of patients in the clinical studies had a prior episode of acute cystitis within the preceding year), no known structural abnormalities, no clinical or laboratory evidence of hepatic dysfunction, and no known or suspected CNS disorders, such as epilepsy, or other factors which would predispose to seizures. In these studies, the following clinical success (resolution of symptoms) and microbiologic eradication rates were obtained.
Treatment Arm Treatment
Duration
(days)Microbiologic Eradication Rate Clinical Success
RateOutcome (based on difference in
microbiologic eradication rates 5-11 days post therapy)5-11 days post therapy Study day
12-21Fosfomycin 1 630/771
(82%)591/771
(77%)542/771
(70%)Ciprofloxacin 7 219/222
(98%)219/222
(98%)213/222
(96%)Fosfomycin inferior to ciprofloxacin Trimethoprim/
sulfamethoxazole10 194/197
(98%)194/197
(98%)186/197
(94%)Fosfomycin inferior to trimethoprim/
sulfamethoxazoleNitrofurantoin 7 180/238
(76%)180/238
(76%)183/238
(77%)Fosfomycin equivalent to
nitrofurantoinPathogen Fosfomycin 3 gram singledose Ciprofloxacin 250 mg
bid x 7 daysTrimethoprim/
sulfamethoxazole 160 mg/800 mg bid x 10 daysNitrofurantoin
100 mg
bid x 7 daysE. coli 509/644 (79%) 184/187
(98%)171/174
(98%)146/187
(78%)E. faecalis 10/10 (100%) 0/0 4/4
(100%)1/2
(50%) -
REFERENCES
1. National Committee for Clinical Laboratory Standards, Methods for Dilution. Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically – Third Edition; Approved Standard NCCLS Document M7-A3, Vol. 13, No. 25 NCCLS, Villanova, PA, December, 1993.
2. National Committee for Clinical Laboratory Standards, Performance Standard for Antimicrobial Disk Susceptibility Tests – Fifth Edition; Approved Standard NCCLS Document M2-A5, Vol. 13, No. 24 NCCLS, Villanova, PA, December, 1993.
Manufactured by:
Zambon Switzerland Ltd.
A Subsidiary of Zambon S.p.A.Via Industria 13
6814 Cadempino, SwitzerlandMade in Switzerland
Distributed by:
Zambon USA Ltd.One Broadway, 14th Floor, Cambridge MA 02142
Zambon TM and its designs are trademarks of Zambon S.p.A.
Rev. June 2021

-
INFORMATION FOR PATIENTS
Fosfomycin Tromethamine
Granules for oral solution
(equivalent to 3 grams of fosfomycin)
Information for patients
After taking fosfomycin tromethaminegranules for oral solution
you should see improvement in your symptomsin 2 to 3 days.
If your symptoms do not improve by the fourth day,
you should contact your healthcare professional.2
This booklet provides information on fosfomycin tromethamine granules for oral solution, the medication your doctor has prescribed for your bladder infection (acute cystitis), as well as background on the infection itself. It is important to know that, although your therapy is completed when you take the single dose of fosfomycin tromethamine granules for oral solution, it may take 2 to 3 days for symptoms to improve. If your symptoms do not improve by the fourth day, you should contact your healthcare professional.
3
What is fosfomycin tromethamine granules for oral solution for?
Fosfomycin tromethamine granules for oral solution is for the treatment of acute cystitis in women. Acute cystitis, also known as bladder infection, is a type of uncomplicated urinary tract infection. Acute cystitis is caused by bacteria entering your bladder (which is normally bacteria free) resulting in irritation to your bladder lining. Fosfomycin tromethamine granules for oral solution is a bladder-specific antibiotic prescribed to kill the bacteria causing your infection. As the bacteria die, your bladder lining begins to heal.
4
How does fosfomycin tromethamine granules for oral solution work?
Fosfomycin tromethamine granules for oral solution starts working to kill bacteria within hours after taking it. Although you only take fosfomycin tromethamine granules for oral solution once, fosfomycin tromethamine granules for oral solution is designed to stay in your bladder for more than 3 days, continuing to kill the bacteria. Therefore, after taking fosfomycin tromethamine granules for oral solution, you should see improvement in your symptoms in 2 to 3 days. If your symptoms do not improve by the fourth day, you should contact your healthcare professional.
5
What is in the sachet (packet)?
The sachet packet contains white granules of fosfomycin tromethamine granules for oral solution (fosfomycin tromethamine), an antibiotic, along with a small amount of sweetener (saccharin and sucrose) and orange and mandarin flavoring.
6
Can I take fosfomycin tromethamine granules for oral solution if I am pregnant or breast-feeding?
Adequate and well-controlled studies involving the use of fosfomycin tromethamine granules for oral solution in pregnant and breast-feeding women have not been conducted. If your physician feels it is clearly needed, fosfomycin tromethamine granules for oral solution can be taken. Please inform your healthcare provider if you know you are pregnant or are breast-feeding.
7
How do I take fosfomycin tromethamine granules for oral solution?
1. Empty entire contents of fosfomycin tromethamine granules for oral solution sachet into 3 to 4 ounces of water (½ cup), and stir to dissolve. Do not use hot water.
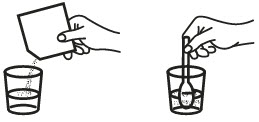
2. Take immediately after dissolving in water, making sure to drink complete contents of glass.
8
Is this all I need to do?
Yes. One sachet of fosfomycin tromethamine granules for oral solution is a complete therapy for treating your bladder infection. The medicine in fosfomycin tromethamine granules for oral solution goes directly to your bladder and stays there for several days to kill the bacteria causing your infection.
9
Does fosfomycin tromethamine granules for oral solution always work?
No antibiotic works 100% of the time. In controlled clinical trials, using strict criteria for evaluation, approximately three quarters of patients were cured after single dose treatment with fosfomycin tromethamine granules for oral solution. This cure rate was equivalent to that for patients treated with Macrobid®**, and somewhat lower than for patients treated with Cipro®† and with Bactrim®††.
10
Will I experience side effects?
Because fosfomycin tromethamine granules for oral solution goes right to the site of your infection, side effects from the drug are uncommon. Less than 1 patient in 10 (9.0%) taking fosfomycin tromethamine granules for oral solution may experience diarrhea. Other infrequently seen side effects are vaginitis (5.5%), nausea (4.1%), headache (3.9%), dizziness (1.3%), tiredness (1.1%), and indigestion (1.1%).
11
What if I am allergic to fosfomycin tromethamine granules for oral solution?
Fosfomycin tromethamine granules for oral solution is not related to penicillin or other antibiotics that commonly cause allergic reactions. If you have a history of allergy to antibiotics, you should be able to take fosfomycin tromethamine granules for oral solution on your doctor’s advice.
12
What about taking fosfomycin tromethamine granules for oral solution with other drugs?
Fosfomycin tromethamine granules for oral solution has very few interactions with other drugs. If you are currently taking a gastrointestinal drug called Reglan®* (metoclopramide hydrochloride), you should not take fosfomycin tromethamine granules for oral solution unless you have discussed this with your doctor.
13
Some other precautions:
Do not take this or any other medication if it was prescribed for someone else.
KEEP FOSFOMYCIN TROMETHAMINE GRANULES FOR ORAL SOLUTION OUT OF THE REACH OF CHILDREN.
Do not use after the expiration date printed on the box.
*Reglan is a registered trademark of A. H. Robins Company.
**Macrobid is a registered trademark of Procter & Gamble.
†Cipro is a registered trademark of Bayer Corporation.
††Bactrim is a registered trademark of Roche Pharmaceuticals.14
What is acute cystitis?
Acute cystitis is the common name for uncomplicated urinary tract infection. It is an infection of the urinary bladder that causes the bladder lining to become inflamed or irritated.
15
What causes acute cystitis?
Acute cystitis is usually caused by bacteria that enter the bladder. Bacteria can travel from the anal area to collect in the vagina and then enter the urethra to finally reach the bladder.
16
What are the usual symptoms?
Typical symptoms include frequent urination, painful urination, and a bladder that never feels totally empty. Other symptoms include pain in the pubic area.
17
Is acute cystitis dangerous?
Normally it is not, unless the bacteria in the urine causing it have traveled upward to infect the kidneys. Your doctor has prescribed fosfomycin tromethamine granules for oral solution to prevent this from occurring.
18
How is acute cystitis diagnosed and treated?
Acute cystitis is diagnosed on the basis of your symptoms and/or a urine culture to identify the responsible bacteria. It is most often treated with antibiotics.
19
Is there something I can take to relieve my pain?
There are medications available in addition to your primary therapy, fosfomycin tromethamine granules for oral solution, that your healthcare provider can recommend. Talk to your healthcare provider about additional medication.
20
Is there anything I can do to avoid acute cystitis?
Bladder infections that occur repeatedly in women are often associated with sexual activity and/or certain methods of contraception. Urinating immediately and completely after intercourse can help flush out bacteria that may have been accidentally forced into the urethra. Women who use a diaphragm with spermicidal jelly or use spermicidal foam with a condom should consider another form of contraception if they continue to experience infections.
21
You should also wipe from front to back to avoid contaminating the urethral entrance with bacteria from the bowels. You should discuss these and other ways to prevent bladder infections with your healthcare provider.
22
DO NOT TAKE THIS OR ANY MEDICATION IF IT WAS PRESCRIBED FOR SOMEONE ELSE.
FOSFOMYCIN TROMETHAMINE GRANULES FOR ORAL SOLUTION IS NOT RECOMMENDED FOR PATIENTS UNDER 18 YEARS OF AGE.
KEEP FOSFOMYCIN TROMETHAMINE GRANULES FOR ORAL SOLUTION OUT OF REACH OF CHILDREN.
DO NOT USE AFTER EXPIRATION DATE.
Manufactured by:
Zambon Switzerland Ltd.
A Subsidiary of Zambon S.p.A.
Via Industria 13
6814 Cadempino, SwitzerlandDistributed by:
Zambon USA Ltd.
One Broadway, 14th Floor, Cambridge MA 02142Zambon TM and its designs are trademarks of Zambon S.p.A.
Made in Switzerland
Zambon USA Ltd.
06/2021
-
PRINCIPAL DISPLAY PANEL
NDC: 82036-4274-1
Fosfomycin Tromethamine
Granules for oral solution
(equivalent to 3 grams of fosfomycin)Rx only
CONTENTS: 1 single dose of fosfomycin tromethamine
Good for one complete course of therapy
-
INGREDIENTS AND APPEARANCE
FOSFOMYCIN TROMETHAMINE
fosfomycin tromethamine powderProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 82036-4274 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOSFOMYCIN TROMETHAMINE (UNII: 7FXW6U30GY) (FOSFOMYCIN - UNII:2N81MY12TE) FOSFOMYCIN 3 g Inactive Ingredients Ingredient Name Strength TANGERINE (UNII: KH3E3096OO) ORANGE (UNII: 5EVU04N5QU) SACCHARIN (UNII: FST467XS7D) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82036-4274-1 1 in 1 CARTON 08/25/2021 1 1 in 1 DOSE PACK; Type 0: Not a Combination Product 2 NDC: 82036-4274-8 1 in 1 PACKET; Type 0: Not a Combination Product 08/25/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA050717 08/25/2021 Labeler - Zambon USA, Ltd (118086168) Establishment Name Address ID/FEI Business Operations Zambon Switzerland Ltd 481761344 manufacture(82036-4274)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.