IXOBA M- moxifloxacin 0.5%, ketorolac 0.5%, prednisolone acetate 1% kit
IXOBA M by
Drug Labeling and Warnings
IXOBA M by is a Prescription medication manufactured, distributed, or labeled by Brisk Pharmaceuticals, Inc., Unit Dose Solutions, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Rx Only
NDC: 73614-454-03
For Use in Eyes Only
IXOBA M
Each Pack Contains:
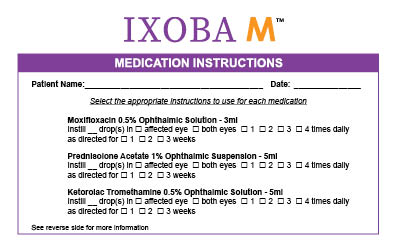
Moxifloxacin 0.5% Ophthalmic Solution - 3ml
Ketorolac 0.5% Ophthalmic Solution - 5ml
Prednisolone Acetate 1% Ophthalmic Suspension - 5ml
Brisk Pharmaceuticals
-
DESCRIPTION
See Instructions on the bottom of the package
What is Ixoba M used for: Ixoba M is a convenient pack containing 3 ophthalmic medication bottles.
How Supplied: Ixoba M is supplied as a co-pack containing Moxifloxacin 0.5% Ophthalmic Suspension 3ml bottle, Ketorolac 0.5% Ophthalmic Suspension 5ml bottle, Prednisolone Acetate 1% Ophthalmic Suspen-sion 5ml bottle.
Storage: Store at 20C to 25C (68F-77F). Protect from light.
Please read the leaflet inside each bottle for ‘full prescribing information’ about that medication.
Patient Counseling Information:
Risk of Contamination: Do not touch the dropper tip to any surface to avoid contaminating the contents by common bacteria known to cause ocular infections.
Concomitant Use of Contact Lenses: Do not administer Moxifloxacin Ophthalmic Suspension, Ketorolac Ophthalmic Suspension or Prednisolone Acetate Ophthalmic Suspension while wearing contact lenses.
Concomitant Topical Ocular Therapy: If more than one topical ophthalmic medication is being used, the medications should be administered at least 5 minutes apart.
For questions on Ixoba M, please call your Ophthalmologist Office or Ixoba Assist at 469-342-1471.
-
DESCRIPTION
Keep out of the reach of children.
TAMPER EVIDENT: Do not use the individual eye drops inside the pack if the seal on its carton is broken or missing.
Lot: See the lot number on each individual bottle inside the pack.
Exp: See the expiration date on each individual bottle inside the pack.
Packaged By: Unit Dose Solutions Inc., Morrisville, NC 27560
Packaged for: Brisk Pharmaceuticals, Dallas, TX 75217
- PATIENT MEDICATION INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IXOBA M
moxifloxacin 0.5%, ketorolac 0.5%, prednisolone acetate 1% kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73614-454 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73614-454-03 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 08/26/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 5 mL Part 2 1 BOTTLE 5 mL Part 3 1 BOTTLE 3 mL Part 1 of 3 KETOROLAC TROMETHAMINE
ketorolac tromethamine solutionProduct Information Item Code (Source) NDC: 61314-126 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOROLAC TROMETHAMINE (UNII: 4EVE5946BQ) (KETOROLAC - UNII:YZI5105V0L) KETOROLAC 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076583 11/05/2009 Part 2 of 3 PREDNISOLONE ACETATE
prednisolone acetate suspension/ dropsProduct Information Item Code (Source) NDC: 60758-119 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PREDNISOLONE ACETATE (UNII: 8B2807733D) (PREDNISOLONE - UNII:9PHQ9Y1OLM) PREDNISOLONE ACETATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CITRATE (UNII: 1Q73Q2JULR) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) SODIUM BISULFITE (UNII: TZX5469Z6I) SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BORIC ACID (UNII: R57ZHV85D4) HYPROMELLOSES (UNII: 3NXW29V3WO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA017011 08/19/1997 Part 3 of 3 MOXIFLOXACIN
moxifloxacin solution/ dropsProduct Information Item Code (Source) NDC: 68180-422 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIFLOXACIN HYDROCHLORIDE MONOHYDRATE (UNII: B8956S8609) (MOXIFLOXACIN - UNII:U188XYD42P) MOXIFLOXACIN 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow (Yellow Colored Transparent) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202867 07/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/26/2021 Labeler - Brisk Pharmaceuticals, Inc. (117250794) Establishment Name Address ID/FEI Business Operations Unit Dose Solutions, Inc 360804194 repack(73614-454)
Trademark Results [IXOBA M]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IXOBA M 88783511 not registered Live/Pending |
Brisk Pharmaceuticals, Inc. 2020-02-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.