These highlights do not include all the information needed to use POTASSIUM CITRATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for POTASSIUM CITRATE EXTENDED-RELEASE TABLETS. POTASSIUM CITRATE extended-release tablets, for oral use Initial U.S. Approval: 1985
Potassium Citrate by
Drug Labeling and Warnings
Potassium Citrate by is a Prescription medication manufactured, distributed, or labeled by Bionpharma Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
POTASSIUM CITRATE- potassium citrate tablet, extended release
Bionpharma Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use POTASSIUM CITRATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for POTASSIUM CITRATE EXTENDED-RELEASE TABLETS.
POTASSIUM CITRATE extended-release tablets, for oral use Initial U.S. Approval: 1985 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATIONObjective: To restore normal urinary citrate (greater than 320 mg/day and as close to the normal mean of 640 mg/day as possible), and to increase urinary pH to a level of 6.0 to 7.0.
DOSAGE FORMS AND STRENGTHSTablets: 10 mEq and 15 mEq (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Bionpharma Inc. at 1-888-235-BION or 1-888-235-2466 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSThe following drug interactions may occur with potassium citrate:
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION. Revised: 1/2022 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Renal Tubular Acidosis (RTA) with Calcium Stones
Potassium citrate extended-release tablets are indicated for the management of renal tubular acidosis [see Clinical Studies (14.1)].
1.2 Hypocitraturic Calcium Oxalate Nephrolithiasis of any Etiology
Potassium citrate extended-release tablets are indicated for the management of hypocitraturic calcium oxalate nephrolithiasis [see Clinical Studies (14.2)].
1.3 Uric Acid Lithiasis with or without Calcium Stones
Potassium citrate extended-release tablets are indicated for the management of uric acid lithiasis with or without calcium stones [see Clinical Studies (14.3)].
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Instructions
Treatment with extended release potassium citrate should be added to a regimen that limits salt intake (avoidance of foods with high salt content and of added salt at the table) and encourages high fluid intake (urine volume should be at least two liters per day). The objective of treatment with potassium citrate extended-release tablets is to provide potassium citrate in sufficient dosage to restore normal urinary citrate (greater than 320 mg/day and as close to the normal mean of 640 mg/day as possible), and to increase urinary pH to a level of 6.0 or 7.0.
Monitor serum electrolytes (sodium, potassium, chloride and carbon dioxide), serum creatinine and complete blood counts every four months and more frequently in patients with cardiac disease, renal disease or acidosis. Perform electrocardiograms periodically. Treatment should be discontinued if there is hyperkalemia, a significant rise in serum creatinine or a significant fall in blood hematocrit or hemoglobin.
2.2 Severe Hypocitraturia
In patients with severe hypocitraturia (urinary citrate less than 150 mg/day), therapy should be initiated at a dosage of 60 mEq/day (30 mEq two times/day or 20 mEq three times/day with meals or within 30 minutes after meals or bedtime snack). Twenty-four-hour urinary citrate and/or urinary pH measurements should be used to determine the adequacy of the initial dosage and to evaluate the effectiveness of any dosage change. In addition, urinary citrate and/or pH should be measured every four months. Doses of potassium citrate greater than 100 mEq/day have not been studied and should be avoided.
2.3 Mild to Moderate Hypocitraturia
In patients with mild to moderate hypocitraturia (urinary citrate greater than 150 mg/day) therapy should be initiated at 30 mEq/day (15 mEq two times/day or 10 mEq three times/day with meals or within 30 minutes after meals or bedtime snack). Twenty-four-hour urinary citrate and/or urinary pH measurements should be used to determine the adequacy of the initial dosage and to evaluate the effectiveness of any dosage change. Doses of potassium citrate greater than 100 mEq/day have not been studied and should be avoided.
4 CONTRAINDICATIONS
Potassium citrate extended-release tablets are contraindicated:
- In patients with hyperkalemia (or who have conditions predisposing them to hyperkalemia), as a further rise in serum potassium concentration may produce cardiac arrest. Such conditions include: chronic renal failure, uncontrolled diabetes mellitus, acute dehydration, strenuous physical exercise in unconditioned individuals, adrenal insufficiency, extensive tissue breakdown or the administration of a potassium-sparing agent (such as triamterene, spironolactone or amiloride).
- In patients in whom there is cause for arrest or delay in tablet passage through the gastrointestinal tract, such as those suffering from delayed gastric emptying, esophageal compression, intestinal obstruction or stricture, or those taking anticholinergic medication.
- In patients with peptic ulcer disease because of its ulcerogenic potential.
- In patients with active urinary tract infection (with either urea-splitting or other organisms, in association with either calcium or struvite stones). The ability of potassium citrate to increase urinary citrate may be attenuated by bacterial enzymatic degradation of citrate. Moreover, the rise in urinary pH resulting from potassium citrate extended-release tablets therapy might promote further bacterial growth.
- In patients with renal insufficiency (glomerular filtration rate of less than 0.7 mL/kg/min), because of the danger of soft tissue calcification and increased risk for the development of hyperkalemia.
5 WARNINGS AND PRECAUTIONS
5.1 Hyperkalemia
In patients with impaired mechanisms for excreting potassium, potassium citrate administration can produce hyperkalemia and cardiac arrest. Potentially fatal hyperkalemia can develop rapidly and be asymptomatic. The use of potassium citrate extended-release tablets in patients with chronic renal failure, or any other condition which impairs potassium excretion such as severe myocardial damage or heart failure, should be avoided. Closely monitor for signs of hyperkalemia with periodic blood tests and ECGs.
5.2 Gastrointestinal Lesions
Solid dosage forms of potassium chlorides have produced stenotic and/or ulcerative lesions of the small bowel and deaths. These lesions are caused by a high local concentration of potassium ions in the region of the dissolving tablets, which injured the bowel. In addition, perhaps because wax-matrix preparations are not enteric-coated and release some of their potassium content in the stomach, there have been reports of upper gastrointestinal bleeding associated with these products. The frequency of gastrointestinal lesions with wax-matrix potassium chloride products is estimated at one per 100,000 patient-years. Experience with potassium citrate extended-release tablets is limited, but a similar frequency of gastrointestinal lesions should be anticipated.
If there is severe vomiting, abdominal pain or gastrointestinal bleeding, potassium citrate extended-release tablets should be discontinued immediately and the possibility of bowel perforation or obstruction investigated.
6 ADVERSE REACTIONS
6.1 Postmarketing Experience
Some patients may develop minor gastrointestinal complaints during potassium citrate therapy, such as abdominal discomfort, vomiting, diarrhea, loose bowel movements or nausea. These symptoms are due to the irritation of the gastrointestinal tract and may be alleviated by taking the dose with meals or snacks, or by reducing the dosage. Patients may find intact matrices in their feces.
7 DRUG INTERACTIONS
7.1 Potential Effects of Potassium Citrate on Other Drugs
Potassium-sparing Diuretics:Concomitant administration of potassium citrate and a potassium-sparing diuretic (such as triamterene, spironolactone or amiloride) should be avoided since the simultaneous administration of these agents can produce severe hyperkalemia.
7.2 Potential Effects of Other Drugs on Potassium Citrate
Drugs that slow gastrointestinal transit time:These agents (such as anticholinergics) can be expected to increase the gastrointestinal irritation produced by potassium salts.
7.3 Renin-Angiotensin-Aldosterone System Inhibitors
Drugs that inhibit the renin-angiotensin-aldosterone system (RAAS) including angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), spironolactone, eplerenone, or aliskiren produce potassium retention by inhibiting aldosterone production. Closely monitor potassium in patients receiving concomitant RAAS therapy.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted. It is also not known whether potassium citrate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Potassium citrate extended-release tablets should be given to a pregnant woman only if clearly needed.
10 OVERDOSAGE
Treatment of Overdosage:The administration of potassium salts to persons without predisposing conditions for hyperkalemia rarely causes serious hyperkalemia at recommended dosages. It is important to recognize that hyperkalemia is usually asymptomatic and may be manifested only by an increased serum potassium concentration and characteristic electrocardiographic changes (peaking of T-wave, loss of P-wave, depression of S-T segment and prolongation of the QT interval). Late manifestations include muscle paralysis and cardiovascular collapse from cardiac arrest.
Treatment measures for hyperkalemia include the following:
- Patients should be closely monitored for arrhythmias and electrolyte changes.
- Elimination of medications containing potassium and of agents with potassium sparing properties such as potassium-sparing diuretics, ARBs, ACE inhibitors, NSAIDs, certain nutritional supplements and many others.
- Elimination of foods containing high levels of potassium such as almonds, apricots, bananas, beans (lima, pinto, white), cantaloupe, carrot juice (canned), figs, grapefruit juice, halibut, milk, oat bran, potato (with skin), salmon, spinach, tuna and many others.
- Intravenous calcium gluconate if the patient is at no risk or low risk of developing digitalis toxicity.
- Intravenous administration of 300 mL to 500 mL/hr of 10% dextrose solution containing 10 units to 20 units of crystalline insulin per 1,000 mL.
- Correction of acidosis, if present, with intravenous sodium bicarbonate.
- Hemodialysis or peritoneal dialysis.
- Exchange resins may be used. However, this measure alone is not sufficient for the acute treatment of hyperkalemia.
Lowering potassium levels too rapidly in patients taking digitalis can produce digitalis toxicity.
11 DESCRIPTION
Potassium citrate is a citrate salt of potassium. Its molecular formula is K 3C 6H 5O 7 H 2O, and it has the following chemical structure:
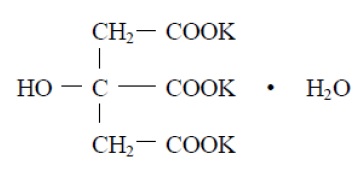
Potassium citrate, USP is a transparent crystals or white, granular powder. It is freely soluble in water; very slightly soluble in alcohol.
Potassium citrate extended-release tablets, USP are yellowish to tan, oral wax-matrix tablets, and contain 10 mEq (1,080 mg) potassium citrate, USP and 15 mEq (1,620 mg) potassium citrate, USP each. Inactive ingredients include carnauba wax and magnesium stearate.
FDA approved dissolution specification differs from the USP dissolution specification.
12 CLINICAL PHARMACOLOGY
When potassium citrate is given orally, the metabolism of absorbed citrate produces an alkaline load. The induced alkaline load in turn increases urinary pH and raises urinary citrate by augmenting citrate clearance without measurably altering ultra-filterable serum citrate. Thus, potassium citrate extended-release tablets therapy appears to increase urinary citrate principally by modifying the renal handling of citrate, rather than by increasing the filtered load of citrate. The increased filtered load of citrate may play some role, however, as in small comparisons of oral citrate and oral bicarbonate, citrate had a greater effect on urinary citrate.
In addition to raising urinary pH and citrate, potassium citrate increases urinary potassium by approximately the amount contained in the medication. In some patients, potassium citrate causes a transient reduction in urinary calcium.
The changes induced by potassium citrate produce urine that is less conducive to the crystallization of stone-forming salts (calcium oxalate, calcium phosphate and uric acid). Increased citrate in the urine, by complexing with calcium, decreases calcium ion activity and thus the saturation of calcium oxalate. Citrate also inhibits the spontaneous nucleation of calcium oxalate and calcium phosphate (brushite).
The increase in urinary pH also decreases calcium ion activity by increasing calcium complexation to dissociated anions. The rise in urinary pH also increases the ionization of uric acid to the more soluble urate ion.
Potassium citrate extended-release tablets therapy does not alter the urinary saturation of calcium phosphate, since the effect of increased citrate complexation of calcium is opposed by the rise in pH-dependent dissociation of phosphate. Calcium phosphate stones are more stable in alkaline urine.
In the setting of normal renal function, the rise in urinary citrate following a single dose begins by the first hour and lasts for 12 hours. With multiple doses the rise in citrate excretion reaches its peak by the third day and averts the normally wide circadian fluctuation in urinary citrate, thus maintaining urinary citrate at a higher, more constant level throughout the day. When the treatment is withdrawn, urinary citrate begins to decline toward the pre-treatment level on the first day.
The rise in citrate excretion is directly dependent on the potassium citrate dosage. Following long-term treatment, potassium citrate at a dosage of 60 mEq/day raises urinary citrate by approximately 400 mg/day and increases urinary pH by approximately 0.7 units.
In patients with severe renal tubular acidosis or chronic diarrheal syndrome where urinary citrate may be very low (<100 mg/day), potassium citrate may be relatively ineffective in raising urinary citrate. A higher dose of potassium citrate may therefore be required to produce a satisfactory citraturic response. In patients with renal tubular acidosis in whom urinary pH may be high, potassium citrate produces a relatively small rise in urinary pH.
14 CLINICAL STUDIES
The pivotal potassium citrate extended-release tablets trials were non-randomized and non-placebo controlled where dietary management may have changed coincidentally with pharmacological treatment. Therefore, the results as presented in the following sections may overstate the effectiveness of the product.
14.1 Renal Tubular Acidosis (RTA) with Calcium Stones
The effect of oral potassium citrate therapy in a non-randomized, non-placebo controlled clinical study of five men and four women with calcium oxalate/calcium phosphate nephrolithiasis and documented incomplete distal renal tubular acidosis was examined. The main inclusion criterion was a history of stone passage or surgical removal of stones during the 3 years prior to initiation of potassium citrate therapy. All patients began alkali treatment with 60 mEq to 80 mEq potassium citrate daily in 3 or 4 divided doses. Throughout treatment, patients were instructed to stay on a sodium restricted diet (100 mEq/day) and to reduce oxalate intake (limited intake of nuts, dark roughage, chocolate and tea). A moderate calcium restriction (400 mg to 800 mg/day) was imposed on patients with hypercalciuria.
X-rays of the urinary tract, available in all patients, were reviewed carefully to determine presence of pre-existing stones, appearance of new stones, or change in the number of stones.
Potassium citrate therapy was associated with inhibition of new stone formation in patients with distal tubular acidosis. Three of the nine patients continued to pass stones during the on-treatment phase. While it is likely that these patients passed pre-existing stones during therapy, the most conservative assumption is that the passed stones were newly formed. Using this assumption, the stone-passage remission rate was 67%. All patients had a reduced stone formation rate. Over the first 2 years of treatment, the on-treatment stone formation rate was reduced from 13±27 to 1±2 per year.
14.2 Hypocitraturic Calcium Oxalate Nephrolithiasis of any Etiology
Eighty-nine patients with hypocitraturic calcium nephrolithiasis or uric acid lithiasis with or without calcium nephrolithiasis participated in this non-randomized, non-placebo controlled clinical study. Four groups of patients were treated with potassium citrate: Group 1 was comprised of 19 patients, 10 with renal tubular acidosis and 9 with chronic diarrheal syndrome, Group 2 was comprised of 37 patients, 5 with uric acid stones alone, 6 with uric acid lithiasis and calcium stones, 3 with type 1 absorptive hypercalciuria, 9 with type 2 absorptive hypercalciuria and 14 with hypocitraturia. Group 3 was comprised of 15 patients with history of relapse on other therapy and Group 4 was comprised of 18 patients, 9 with type 1 absorptive hypercalciuria and calcium stones, 1 with type 2 absorptive hypercalciuria and calcium stones, 2 with hyperuricosuric calcium oxalate nephrolithiasis, 4 with uric acid lithiasis accompanied by calcium stones and 2 with hypocitraturia and hyperuricemia accompanied by calcium stones. The dose of potassium citrate ranged from 30 mEq to 100 mEq per day, and usually was 20 mEq administered orally 3 times daily. Patients were followed in an outpatient setting every 4 months during treatment and were studied over a period from 1 year to 4.33 years. A three-year retrospective pre-study history for stone passage or removal was obtained and corroborated by medical records. Concomitant therapy (with thiazide or allopurinol) was allowed if patients had hypercalciuria, hyperuricosuria or hyperuricemia. Group 2 was treated with potassium citrate alone.
In all groups, treatment that included potassium citrate was associated with a sustained increase in urinary citrate excretion from subnormal values to normal values (400 mg to 700 mg/day), and a sustained increase in urinary pH from 5.6 to 6.0 to approximately 6.5. The stone formation rate was reduced in all groups as shown in Table 1.
Table 1. Effect of Potassium Citrate in Patients with Calcium Oxalate Nephrolithiasis.
|
*Remission defined as “the percentage of patients remaining free of newly formed stones during treatment”. |
||||
| Group | Baseline | On Treatment | Remission* | Any Decrease |
| I (n=19) | 12 ± 30 | 0.9 ± 1.3 | 58% | 95% |
| II (n=37) | 1.2 ± 2 | 0.4 ± 1.5 | 89% | 97% |
| III (n=15) | 4.2 ± 7 | 0.7 ± 2 | 67% | 100% |
| IV (n=18) | 3.4 ± 8 | 0.5 ± 2 | 94% | 100% |
| Total (n=89) | 4.3 ±15 | 0.6 ± 2 | 80% | 98% |
14.3 Uric Acid Lithiasis with or without Calcium Stones
A long-term non-randomized, non-placebo controlled clinical trial with eighteen adult patients with uric acid lithiasis participated in the study. Six patients formed only uric acid stones, and the remaining 12 patients formed mixed stones containing both uric acid and calcium salts or formed both uric acid stones (without calcium salts) and calcium stones (without uric acid) on separate occasions.
Eleven of the 18 patients received potassium citrate alone. Six of the 7 other patients also received allopurinol for hyperuricemia with gouty arthritis, symptomatic hyperuricemia, or hyperuricosuria. One patient also received hydrochlorothiazide because of unclassified hypercalciuria. The main inclusion criterion was a history of stone passage or surgical removal of stones during the 3 years prior to initiation of potassium citrate therapy. All patients received potassium citrate at a dosage of 30 mEq to 80 mEq/day in three-to-four divided doses and were followed every four months for up to 5 years.
While on potassium citrate treatment, urinary pH rose significantly from a low value of 5.3 ± 0.3 to within normal limits (6.2 to 6.5). Urinary citrate which was low before treatment rose to the high normal range and only one stone was formed in the entire group of 18 patients.
16 HOW SUPPLIED/STORAGE AND HANDLING
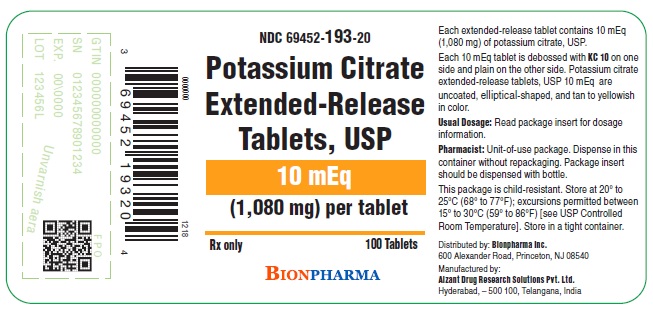
Potassium citrate extended-release tablets, USP 10 mEq are uncoated, tan to yellowish in color, elliptical-shaped, with KC 10debossed on one side and plain on the other, supplied in bottles as:
NDC: 69452-193-20 Bottle of 100 with child-resistant closure
NDC: 69452-193-30 Bottle of 500 with child-resistant closure
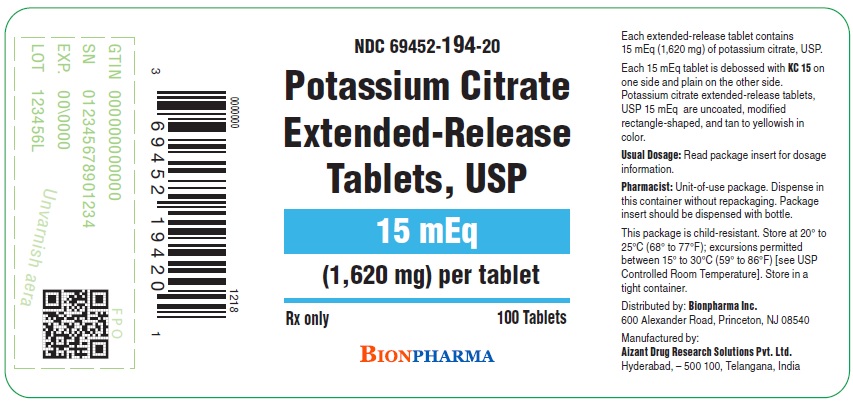
Potassium citrate extended-release tablets, USP 15 mEq are uncoated, tan to yellowish in color, modified rectangle-shaped, with KC 15debossed on one side and plain on the other, supplied in bottles as:
NDC: 69452-194-20 Bottle of 100 with child-resistant closure
NDC: 69452-194-30 Bottle of 500 with child-resistant closure
Storage:Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Store in a tight container.
Keep out of reach of children.
17 PATIENT COUNSELING INFORMATION
17.1 Administration of Drug
Tell patients to take each dose without crushing, chewing or sucking the tablet.
Tell patients to take this medicine only as directed. This is especially important if the patient is also taking both diuretics and digitalis preparations.
Tell patients to check with the doctor if there is trouble swallowing tablets or if the tablet seems to stick in the throat.
Tell patients to check with the doctor at once if tarry stools or other evidence of gastrointestinal bleeding is noticed.
Tell patients that their doctor will perform regular blood tests and electrocardiograms to ensure safety.
Distributed by:
Bionpharma Inc.
600 Alexander Road,
Princeton, NJ 08540
MADE In INDIA
January 2022
FDA-05
PRINCIPAL DISPLAY PANEL - 10 mEq 100 Tablet Bottle Label
NDC: 69452-193-20
Potassium Citrate Extended-Release Tablets, USP
10 mEq
(1,080 mg) per tablet
Rx only
100 Tablets
BIONPHARMA
PRINCIPAL DISPLAY PANEL - 15 mEq 100 Tablet Bottle Label
NDC: 69452-194-20
Potassium Citrate Extended-Release Tablets, USP
15 mEq
(1,620 mg) per tablet
Rx only
100 Tablets
BIONPHARMA
| POTASSIUM CITRATE
potassium citrate tablet, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| POTASSIUM CITRATE
potassium citrate tablet, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bionpharma Inc. (079637826) |
| Registrant - Bionpharma Inc. (079637826) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aizant Drug Research Solutions Pvt. Ltd. | 650372951 | manufacture(69452-193, 69452-194) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.