SEIZALAM- midazolam hydrochloride injection, solution
Seizalam by
Drug Labeling and Warnings
Seizalam by is a Prescription medication manufactured, distributed, or labeled by Meridian Medical Technologies, Inc., Hospira, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SEIZALAMTM safely and effectively. See full prescribing information for SEIZALAMTM.
SEIZALAMTM (midazolam injection), for intramuscular use, CIV
Initial U.S. Approval: 1985INDICATIONS AND USAGE
Seizalam is a benzodiazepine indicated for the treatment of status epilepticus in adults. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 50 mg/10 mL (5 mg/mL) in a multiple-dose vial. (3)
CONTRAINDICATIONS
Hypersensitivity to midazolam. (4)
WARNINGS AND PRECAUTIONS
- Risks of Cardiorespiratory Adverse Reactions: Serious cardiorespiratory adverse reactions have occurred, sometimes resulting in death or permanent neurologic injury, after administration of midazolam. (5.2)
- Other Adverse Reactions: Agitation can occur. (5.3)
- Risks from Concomitant Use of Central Nervous System (CNS) Depressants: May increase risk of hypoventilation, airway obstruction, desaturation, or apnea, and may contribute to profound and/or prolonged drug effect. Practitioners administering Seizalam must have the skills necessary to manage serious cardiorespiratory adverse reactions, including skills in airway management.(5.4)
- Impaired Cognitive Function: Because of partial or complete impairment of recall, patients should not operate hazardous machinery or a motor vehicle until drug effects have subsided. (5.5)
ADVERSE REACTIONS
The most common adverse reactions (incidence >3%) in clinical trials in patients with status epilepticus were upper airway obstruction, agitation, and pyrexia. (6.1)
The most common adverse reaction (incidence >5%) observed in clinical trials for uses other than that for which Seizalam is indicated was decreased tidal volume and/or respiratory rate decrease (11%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or www.meridianmeds.com or FDA at 1-800-FDA-1088 or www.fda.gov.medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Important Administration Instructions
2.3 Monitoring
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
5.2 Risks of Cardiorespiratory Adverse Reactions
5.3 Other Adverse Reactions
5.4 Risks from Concomitant Use of Central Nervous System Depressants
5.5 Impaired Cognitive Function
5.6 Glaucoma
5.7 Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Concomitant Use of Benzodiazepines and Opioids
7.2 Other CNS Depressants and Alcohol
7.3 Cytochrome P450-3A4 Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Congestive Heart Failure
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- BOXED WARNING (What is this?)
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
The recommended dose of Seizalam is 10 mg, administered by intramuscular injection.
2.2 Important Administration Instructions
Seizalam should be administered by a healthcare professional who has had adequate training in the recognition and treatment of status epilepticus.
Seizalam is for intramuscular use only. Inject in the mid-outer thigh (vastus lateralis muscle).
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit [see Dosage Forms and Strengths (3)].
2.3 Monitoring
After administration of Seizalam, continuous monitoring of respiratory and cardiac function is recommended until the patient is stabilized. Serious and life-threatening cardiorespiratory adverse reactions, such as hypoventilation, airway obstruction, apnea, and hypotension have been reported with the use of midazolam. Patients should be monitored in a setting that allows for immediate access to resuscitative drugs. Appropriate resuscitation equipment and personnel trained in their use and skilled in airway management should be available [see Warnings and Precautions (5.2), Adverse Reactions (6.1)].
Observation for signs of cardiorespiratory depression is particularly important in patients with chronic obstructive pulmonary disease (COPD), patients 60 or more years of age, and patients who have received concomitant narcotics or other central nervous system (CNS) depressants.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including Seizalam, and opioids may result in profound sedation, respiratory depression, coma, and death. If a decision is made to use midazolam concomitantly with opioids, monitor patients closely for respiratory depression and sedation [see Drug Interactions (7.1)].
Practitioners administering Seizalam must have the skills necessary to manage serious cardiorespiratory adverse reactions, including skills in airway management.
5.2 Risks of Cardiorespiratory Adverse Reactions
Serious cardiorespiratory adverse reactions have occurred after administration of midazolam. These have included respiratory depression, airway obstruction, oxygen desaturation, apnea, respiratory arrest and/or cardiac arrest, sometimes resulting in death or permanent neurologic injury. There have also been rare reports of hypotensive episodes requiring treatment during or after diagnostic or surgical manipulations, particularly in patients with hemodynamic instability. Hypotension occurs more frequently in patients premedicated with a narcotic. The danger of hypoventilation, airway obstruction, or apnea is greater in elderly patients and those with chronic disease states or decreased pulmonary reserve [see Use in Specific Populations (8.5, 8.7)]; patients with COPD are highly sensitive to the respiratory depressant effect of midazolam. Seizalam should be administered with caution to patients in shock or coma with depression of vital signs.
Practitioners administering Seizalam must have the skills necessary to manage serious cardiorespiratory adverse reactions, including skills in airway management.
5.3 Other Adverse Reactions
Reactions such as agitation, involuntary movements (including tonic/clonic movements and muscle tremor), hyperactivity, and combativeness have been reported with midazolam when used for sedation. These reactions may be caused by inadequate or excessive dosing or improper administration of midazolam; however, consideration should be given to the possibility of cerebral hypoxia or true paradoxical reactions. Agitation also occurred in the randomized controlled clinical study of Seizalam in patients with status epilepticus [see Adverse Reactions (6.1)].
5.4 Risks from Concomitant Use of Central Nervous System Depressants
Concomitant use of barbiturates, alcohol or other central nervous system depressants may increase the risk of hypoventilation, airway obstruction, desaturation, or apnea and may contribute to profound and/or prolonged drug effect. Seizalam should be administered with caution to patients in acute alcohol intoxication with depression of vital signs. Narcotic premedication also depresses the ventilatory response to carbon dioxide stimulation.
The efficacy and safety of midazolam in clinical use are functions of the dose administered, the clinical status of the individual patient, and the use of concomitant medications capable of depressing the central nervous system (CNS). Anticipated effects range from mild sedation to deep levels of sedation virtually equivalent to a state of general anesthesia where the patient may require external support of vital functions. Practitioners administering Seizalam must have the skills necessary to manage serious cardiorespiratory adverse reactions, including skills in airway management. For information regarding withdrawal, see Drug Abuse and Dependence (9.3).
5.5 Impaired Cognitive Function
Midazolam is associated with a high incidence of partial or complete impairment of recall for several hours following an administered dose. Gross tests of recovery from the effects of midazolam cannot be relied upon to predict reaction time under stress. It is recommended that no patient operate hazardous machinery or a motor vehicle until the effects of the drug, such as drowsiness, have subsided, and as their medical condition permits.
5.6 Glaucoma
Benzodiazepines, including Seizalam, can increase intraocular pressure in patients with glaucoma. Measurements of intraocular pressure in patients without eye disease show a moderate lowering following induction with midazolam; patients with glaucoma have not been studied. Patients with open-angle glaucoma may need to have their ophthalmologic status evaluated following treatment with Seizalam. Seizalam is not recommended in patients with narrow-angle glaucoma.
5.7 Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative
Seizalam is not approved for use in neonates or infants. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low birth weight infants treated with benzyl alcohol-preserved drugs, including Seizalam. The “gasping syndrome” is characterized by CNS depression, metabolic acidosis, and gasping respirations. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known (Seizalam contains 10 mg of benzyl alcohol per mL) [see Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections:
- Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
- Risks of Cardiorespiratory Adverse Reactions [see Warnings and Precautions (5.2)]
- Other Adverse Reactions [see Warnings and Precautions (5.3)]
- Risks from Concomitant Use of Central Nervous System Depressants [see Warnings and Precautions (5.4)]
- Glaucoma [see Warnings and Precautions (5.6)]
- Risk of Serious Adverse Reactions in Infants due to Benzyl Alcohol Preservative [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in the Controlled Study of Intramuscular Midazolam in Patients with Status Epilepticus
In a double-blind, randomized, active-controlled clinical study, 448 patients were assigned to receive intramuscular (IM) midazolam via an autoinjector, and 445 were assigned to receive intravenous (IV) lorazepam. Approximately 45% of patients were female, and the mean age was 43 years. Patients were administered treatment by a healthcare professional (e.g., paramedic) prior to arrival at a hospital.
Table 1 lists the adverse reactions occurring in 2% or more of the IM midazolam-treated patients and at a rate greater than the IV lorazepam-treated patients.
Table 1 Adverse Reactions in 2% or More of IM Midazolam-Treated Patients and More Frequent than in IV Lorazepam-Treated Patients in Out of Hospital Treatment of Status Epilepticus Adverse Reaction IM Midazolam
N=448
(%)IV Lorazepam
N=445
(%)Upper airway obstruction 5 3 Agitation 4 3 Pyrexia 4 3 Mental status changes 3 2 Postictal state 3 2 Acute renal failure 2 1 Adverse Reactions in Other Midazolam Studies
Fluctuations in vital signs were the most frequently seen findings following parenteral administration of midazolam in adults for uses other than that for which Seizalam is indicated, and included decreased tidal volume and/or respiratory rate decrease [11% of patients following intramuscular administration], as well as variations in blood pressure and pulse rate. The majority of serious adverse effects, particularly those associated with oxygenation and ventilation, have been reported when midazolam was administered with other medications capable of depressing the CNS. The incidence of such events was higher in patients undergoing procedures involving the airway without the protective effect of an endotracheal tube (e.g., upper endoscopy and dental procedures).
The following additional adverse reactions were reported after intramuscular administration in adults: Headache (1.3%), and local effects at the IM injection site including pain (3.7%), induration (0.5%), redness (0.5%), and muscle stiffness (0.3%).
-
7 DRUG INTERACTIONS
7.1 Effect of Concomitant Use of Benzodiazepines and Opioids
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids. Monitor patients closely for respiratory depression and sedation.
7.2 Other CNS Depressants and Alcohol
The sedative effect of Seizalam is accentuated by concomitantly administered medication that depresses the central nervous system, particularly opioids (e.g., morphine, meperidine, and fentanyl), secobarbital, and droperidol, and also by alcohol [see Warnings and Precautions (5.1, 5.2, 5.4)].
7.3 Cytochrome P450-3A4 Inhibitors
Caution is advised when Seizalam is administered concomitantly with drugs that are known to inhibit the P450-3A4 enzyme system (e.g., cimetidine, erythromycin, diltiazem, verapamil, ketoconazole, and itraconazole). These drug interactions may result in prolonged sedation caused by a decrease in plasma clearance of midazolam [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), such as Seizalam, during pregnancy. Encourage women who are taking Seizalam during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) pregnancy registry by calling 1-888-233-2334 or visiting http://www.aedpregnancyregistry.org/.
Risk Summary
There are no adequate and well-controlled studies of Seizalam in pregnant women. Available data suggest that the class of benzodiazepines is not associated with marked increases in risk for congenital anomalies. Although some early epidemiological studies suggested a relationship between benzodiazepine use in pregnancy and congenital anomalies such as cleft lip and or palate, these studies had considerable limitations. More recently completed studies of benzodiazepine use in pregnancy have not consistently documented elevated risks for specific congenital anomalies. There is insufficient evidence to assess the effect of benzodiazepine pregnancy exposure on neurodevelopment.
There are clinical considerations regarding exposure to benzodiazepines during the second and third trimester of pregnancy or immediately prior to or during childbirth. These risks include decreased fetal movement and/or fetal heart rate variability, “floppy infant syndrome,” dependence, and withdrawal (see Clinical Considerations and Human Data).
Administration of midazolam to rats and rabbits during the period of organogenesis or to rats during late pregnancy and throughout lactation at doses greater than those used clinically did not result in adverse effects on development (see Animal Data). Data for other benzodiazepines suggest the possibility of increased neuronal cell death and long-term effects on neurobehavioral and immunological function based on findings in animals following prenatal or early postnatal exposure at clinically relevant doses. Seizalam should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus. Advise a pregnant woman and women of childbearing age of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Infants born to mothers who have taken benzodiazepines during the later stages of pregnancy can develop dependence, and subsequently withdrawal, during the postnatal period. Clinical manifestations of withdrawal or neonatal abstinence syndrome may include hypertonia, hyperreflexia, hypoventilation, irritability, tremors, diarrhea, and vomiting. These complications can appear shortly after delivery to 3 weeks after birth, and persist from hours to several months, depending on the degree of dependence and the pharmacokinetic profile of the benzodiazepine. Symptoms may be mild and transient or severe. Standard management for neonatal withdrawal syndrome has not yet been defined. Observe newborns who are exposed to Seizalam in utero during the later stages of pregnancy for symptoms of withdrawal and manage accordingly.
Labor and Delivery
Administration of benzodiazepines immediately prior to or during childbirth can result in a floppy infant syndrome, which is characterized by lethargy, hypothermia, hypotonia, respiratory depression, and difficulty feeding. Floppy infant syndrome occurs mainly within the first hours after birth and may last up to 14 days. Observe exposed newborns for these symptoms and manage accordingly.
Data
Human Data
Congenital Anomalies
Although there are no adequate and well controlled studies of Seizalam in pregnant women, there is information about benzodiazepines as a class. Dolovich et al. published a meta-analysis of 23 studies that examined the effects of benzodiazepine exposure during the first trimester of pregnancy. Eleven of the 23 studies included in the meta-analysis considered the use of chlordiazepoxide and diazepam and not other benzodiazepines. The authors considered case-control and cohort studies separately. The data from the cohort studies did not suggest an increased risk for major malformations (OR 0.90; 95% CI 0.61—1.35) or for oral cleft (OR 1.19; 95% CI 0.34—4.15). The data from the case-control studies suggested an association between benzodiazepines and major malformations (OR 3.01, 95% CI 1.32—6.84) and oral cleft (OR 1.79; 95% CI 1.13—2.82). The limitations of this meta-analysis included the small number of reports included in the analysis, and that most cases for analyses of both oral cleft and major malformations came from only three studies. A follow up to that meta-analysis included 3 new cohort studies that examined risk for major malformations and one study that considered cardiac malformations. The authors found no new studies with an outcome of oral clefts. After the addition of the new studies, the odds ratio for major malformations with first trimester exposure to benzodiazepines was 1.07 (95% CI 0.91—1.25).
Neonatal Withdrawal and Floppy Infant Syndrome
Neonatal withdrawal syndrome and symptoms suggestive of floppy infant syndrome associated with administration of benzodiazepines during the later stages of pregnancy and peripartum period have been reported. Findings in published scientific literature suggest that the major neonatal side effects of benzodiazepines include sedation and dependence with withdrawal signs. Data from observational studies suggest that fetal exposure to benzodiazepines is associated with the neonatal adverse events of hypotonia, respiratory problems, hypoventilation, low Apgar score, and neonatal withdrawal syndrome.
Animal Data
When midazolam (0, 0.2, 1, or 4 mg/kg/day) was administered intravenously to pregnant rats during the period of organogenesis, no adverse effects on embryofetal development were observed. The highest dose tested, which was associated with minimal evidence of maternal toxicity, is approximately 4 times the recommended human dose (RHD) of 10 mg based on body surface area (mg/m2).
When midazolam (0, 0.2, 0.6, and 2 mg/kg/day) was administered intravenously to rabbits during the period of organogenesis, no adverse effects on embryofetal development were reported. The high dose, which was not associated with evidence of maternal toxicity, is approximately 4 times the RHD on a mg/m2 basis.
When midazolam (0, 0.2, 1, or 4 mg/kg/day) was administered intravenously to female rats during late gestation and throughout lactation, no clear adverse effects were noted in the offspring. The high dose, which was not associated with evidence of maternal toxicity, is approximately 4 times the RHD on a mg/m2 basis.
In published animal studies, administration of benzodiazepines or other drugs that enhance GABAergic inhibition to neonatal rats has been reported to result in widespread apoptotic neurodegeneration in the developing brain at plasma concentrations relevant for seizure control in humans. The window of vulnerability to these changes in rats (postnatal days 0-14) includes a period of brain development that takes place during the third trimester of pregnancy in humans.
8.2 Lactation
Risk Summary
Midazolam is excreted in human milk. Studies assessing the effects of midazolam in breastfed children or on milk production/excretion have not been performed. Postmarketing experience suggests that breastfed infants of mothers taking benzodiazepines, such as Seizalam, may have effects of lethargy, somnolence and poor sucking. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for midazolam and any potential adverse effects on the breastfed child from midazolam or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Benzodiazepines are not recognized as a treatment for status epilepticus in neonates and should not be used in this population.
Seizalam is not approved for use in neonates or infants. Serious adverse reactions including fatal reactions and the “gasping syndrome” occurred in premature neonates and low-birth-weight infants in the neonatal intensive care unit who received drugs containing benzyl alcohol as a preservative. In these cases, benzyl alcohol dosages of 99 to 234 mg/kg/day produced high levels of benzyl alcohol and its metabolites in the blood and urine (blood levels of benzyl alcohol were 0.61 to 1.378 mmol/L). Additional adverse reactions included gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Preterm, low-birth-weight infants may be more likely to develop these reactions because they may be less able to metabolize benzyl alcohol. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known (Seizalam contains 10 mg of benzyl alcohol per mL) [see Warnings and Precautions (5.7)].
8.5 Geriatric Use
Of the total number of patients from the intent-to-treat (ITT) population in the clinical trial of Seizalam, 14.9 percent were 65 years of age and over, while 8.3 percent were 75 years of age and over.
Geriatric patients may have altered drug distribution; diminished hepatic and/or renal function; longer elimination half-lives for midazolam and its metabolites, and subjects over 70 years of age may be particularly sensitive [see Clinical Pharmacology (12.3)]. Administration of IM midazolam to elderly patients has been associated with rare reports of death under circumstances compatible with cardiorespiratory depression [see Warnings and Precautions (5.2)]. In most of these cases, the patients also received other CNS depressants capable of depressing respiration, especially narcotics [see Warnings and Precautions (5.1, 5.4)]. Close monitoring of geriatric patients is recommended.
8.6 Renal Impairment
Patients with renal impairment may have a slower elimination of midazolam and its metabolites, which may result in prolonged drug exposure [see Clinical Pharmacology (12.3)].
8.7 Congestive Heart Failure
Patients with congestive heart failure eliminate midazolam more slowly, which may result in prolonged drug exposure [see Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Seizalam contains midazolam hydrochloride, a Schedule IV controlled substance.
9.2 Abuse
Midazolam was actively self-administered in primate models used to assess the positive reinforcing effects of psychoactive drugs. Midazolam produced physical dependence of a mild to moderate intensity in cynomolgus monkeys after 5 to 10 weeks of administration.
Available data concerning the drug abuse and dependence potential of midazolam suggest that its abuse potential is at least equivalent to that of diazepam.
9.3 Dependence
Withdrawal symptoms, similar in character to those noted with barbiturates and alcohol (convulsions, hallucinations, tremor, abdominal and muscle cramps, vomiting and sweating), have occurred following abrupt discontinuation of benzodiazepines, including midazolam. Abdominal distention, nausea, vomiting, and tachycardia are prominent symptoms of withdrawal in infants. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Generally milder withdrawal symptoms (e.g., dysphoria and insomnia) have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation should generally be avoided and a gradual dosage tapering schedule followed. There is no consensus in the medical literature regarding tapering schedules; therefore, practitioners are advised to individualize therapy to meet patient's needs. In some case reports, patients who have had severe withdrawal reactions due to abrupt discontinuation of high-dose long-term midazolam, have been successfully weaned off of midazolam over a period of several days.
-
10 OVERDOSAGE
Symptoms
The manifestations of midazolam overdosage reported are similar to those observed with other benzodiazepines, including sedation, somnolence, confusion, impaired coordination, diminished reflexes, coma, and untoward effects on vital signs.
Treatment
Treatment of injectable midazolam overdosage is the same as that followed for overdosage with other benzodiazepines. Respiration, pulse rate, and blood pressure should be monitored and general supportive measures should be employed. Attention should be given to the maintenance of a patent airway and support of ventilation, including administration of oxygen. An intravenous infusion should be started. Should hypotension develop, treatment may include intravenous fluid therapy, repositioning, judicious use of vasopressors appropriate to the clinical situation, if indicated, and other appropriate countermeasures. There is no information as to whether peritoneal dialysis, forced diuresis, or hemodialysis are of any value in the treatment of midazolam overdosage.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. There are anecdotal reports of reversal of adverse hemodynamic responses associated with midazolam following administration of flumazenil to pediatric patients. Prior to the administration of flumazenil, necessary measures should be instituted to secure the airway, assure adequate ventilation, and establish adequate intravenous access. The reversal of benzodiazepine effects may be associated with the onset of seizures in certain high-risk patients. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users. The administration of flumazenil in cases of benzodiazepine overdose can lead to withdrawal and adverse reactions, including increased seizures. Its use in patients with epilepsy is typically not recommended.
-
11 DESCRIPTION
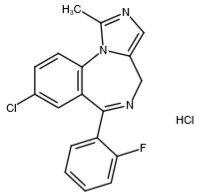
Midazolam is a white to light yellow crystalline compound, insoluble in water. The hydrochloride salt of midazolam, which is formed in situ, is soluble in aqueous solutions. Chemically, midazolam HCl is 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine hydrochloride. Midazolam hydrochloride has the empirical formula C18H13ClFN3HCl, a calculated molecular weight of 362.24 and the following structural formula:
Seizalam is a sterile, nonpyrogenic solution for intramuscular injection. Each mL contains 5 mg midazolam (equivalent to 5.6 mg midazolam hydrochloride) compounded with 1% benzyl alcohol as preservative, 0.01% edetate disodium, and 0.8% sodium chloride. The pH is adjusted to approximately 3 with hydrochloric acid and, if necessary, sodium hydroxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism of action for midazolam in the treatment of status epilepticus is not fully understood, but is thought to involve potentiation of GABAergic neurotransmission resulting from binding at the benzodiazepine site of the GABAA receptor.
12.2 Pharmacodynamics
The effects of midazolam on the CNS are dependent on the dose administered, the route of administration, and the presence or absence of other medications.
12.3 Pharmacokinetics
The pharmacokinetics of midazolam were evaluated in a single dose-escalation trial in healthy subjects; following IM injection of midazolam at doses ranging from fixed total amounts of 5 mg to 30 mg (one half to three times the recommended dose) or body weight based dose of 0.10 mg/kg to 0.49 mg/kg, the overall median time to maximum plasma concentration (Tmax) was observed at approximately 0.5 hours after administration. The rate and extent of systemic exposure, as assessed by maximum plasma concentration (Cmax) and area under the plasma drug concentration-time curve from time 0 to infinity (AUC0-∞), tended to increase proportionally with increasing dose from 5 mg to 30 mg.
Absorption
Following IM administration of a single 10 mg midazolam dose to healthy subjects, midazolam was absorbed with median Tmax (range) of 0.5 (0.25 to 0.5) hours; midazolam mean (±SD) Cmax and AUC0-∞ were 113.9 (±30.9) ng/mL and 402.7 (±97.0) ng∙hr/mL, respectively.
Distribution
The mean (±SD) apparent volume of distribution (Vz/F) of midazolam following a single IM dose of 10 mg midazolam was 2117 (±845.1) mL/kg in healthy subjects.
In humans, midazolam has been shown to cross the placenta and enter into fetal circulation, and has been detected in human milk and CSF [see Use in Specific Populations (8.1, 8.2)].
In adults, midazolam is approximately 97% bound to plasma protein, principally albumin. The 1-hydroxy metabolite is approximately 89% bound to plasma protein.
Elimination
Elimination of the parent drug takes place via hepatic metabolism of midazolam to hydroxylated metabolites that are conjugated and excreted in the urine.
Metabolism
In vitro studies with human liver microsomes indicate that the biotransformation of midazolam is mediated by the cytochrome P450-3A4 (CYP3A4). This enzyme is present in gastrointestinal tract mucosa, as well as in the liver. The 1-hydroxy-midazolam (also termed alpha-hydroxymidazolam) metabolite comprises 60% to 70% of the biotransformation products of midazolam, while 4-hydroxy-midazolam constitutes 5% or less. Small amounts of a dihydroxy derivative have also been detected, but not quantified. The principal urinary excretion products are glucuronide conjugates of the hydroxylated derivatives.
Studies of the intravenous administration of 1-hydroxy-midazolam in humans suggest that 1-hydroxy-midazolam is at least as potent as the parent compound, and may contribute to the net pharmacologic activity of midazolam. In vitro studies have demonstrated that the affinities of 1- and 4-hydroxy-midazolam for the benzodiazepine receptor are approximately 20% and 7%, respectively, relative to midazolam.
Excretion
Following IM administration of 10 mg midazolam, mean (±SD) elimination half-life and apparent total body clearance (CL/F) of midazolam were 4.2 (±1.87) hours and 367.3 (±73.5) mL/hr/kg, respectively.
The principal urinary excretion product is 1-hydroxy-midazolam in the form of a glucuronide conjugate; smaller amounts of the glucuronide conjugates of 4-hydroxy- and dihydroxy-midazolam are detected as well. The amount of midazolam excreted unchanged in the urine after a single IV dose is less than 0.5%. Following a single IV infusion in 5 healthy volunteers, 45% to 57% of the dose was excreted in the urine as the 1-hydroxymethyl midazolam conjugate.
Specific Populations
Changes in the pharmacokinetic profile of midazolam due to drug interactions, physiological variables, etc., may result in changes in the plasma concentration-time profile and pharmacological response to midazolam in these patients. For example, patients with acute renal failure (ARF) appear to have a longer elimination half-life for midazolam [see Use in Specific Populations (8.6)]. In other groups, the relationship between prolonged half-life and duration of effect has not been established.
Obesity
In a study comparing normal (n=20) and obese patients (n=20), the mean half-life was greater in the obese group (5.9 versus 2.3 hours). This was due to an increase of approximately 50% in the volume of distribution (Vd) corrected for total body weight. The clearance was not significantly different between groups.
Geriatric Patients
In three parallel-group studies, the pharmacokinetics of midazolam administered IV or IM were compared in young (mean age 29 years, n=52) and healthy elderly subjects (mean age 73 years, n=53). Plasma half-life was approximately 2-fold higher in the elderly. The mean Vd based on total body weight increased consistently between 15% and 100% in the elderly. The mean CL (total clearance) decreased approximately 25% in the elderly in two studies, and was similar to that of the younger patients in the other [see Use in Specific Populations (8.5)].
Male and Female Patients
No meaningful differences in midazolam exposures (Cmax and AUC) were observed between male and female adults after IM administration.
Patients with Congestive Heart Failure
In patients suffering from congestive heart failure, a 2-fold increase in the elimination half-life, a 25% decrease in the plasma clearance, and a 40% increase in the volume of distribution of midazolam were observed.
Patients with Renal Impairment
Patients with renal impairment may have longer elimination half-lives for midazolam and its metabolites [see Use in Specific Populations (8.6)].
Midazolam and 1-hydroxy-midazolam pharmacokinetics were compared between 6 intensive care unit (ICU) patients who developed acute renal failure (ARF) and a control group of subjects with normal renal function. Midazolam was administered as an IV infusion (5 to 15 mg/hour). Midazolam clearance was reduced (1.9 versus 2.8 mL/min/kg), and the half-life was prolonged (7.6 hours versus 13 hours) in the ARF patients. The renal clearance of the 1-hydroxy-midazolam glucuronide was prolonged in the ARF group (4 versus 136 mL/min), and the half-life was prolonged (12 hours versus >25 hours). Plasma levels accumulated in all ARF patients to about ten times that of the parent drug. The relationship between accumulating metabolite levels and prolonged sedation is unclear.
In a study of chronic renal failure patients (n=15) receiving a single IV dose of midazolam, there was a 2-fold increase in the clearance and volume of distribution, but the half-life remained unchanged. Metabolite levels were not studied.
Patients with Hepatic Impairment
Midazolam pharmacokinetics were studied after a single IV dose (0.075 mg/kg) was administered to 7 patients with biopsy-proven alcoholic cirrhosis and 8 control patients. The mean half-life of midazolam increased 2.5-fold in the patients with cirrhosis. Clearance was reduced by 50% and Vd increased by 20%. In another study in 21 male patients with cirrhosis, without ascites and with normal kidney function as determined by creatinine clearance, no changes in the pharmacokinetics of midazolam or 1-hydroxy-midazolam were observed when compared to healthy individuals. The clinical significance of these findings is unknown.
Drug Interaction Studies
CYP3A4 Inhibitors
Drugs that inhibit the activity of CYP3A4 may inhibit midazolam clearance and elevate midazolam concentrations [see Drug Interactions (7.3)].
- The effect of single oral doses of 800 mg cimetidine and 300 mg ranitidine on steady-state concentrations of oral midazolam was examined in a randomized crossover study (n=8). Cimetidine increased the mean midazolam steady-state concentration from 57 to 71 ng/mL. Ranitidine increased the mean steady-state concentration to 62 ng/mL. No change in choice reaction time or sedation index was detected after dosing with the H2 receptor antagonists.
- In a placebo-controlled study, erythromycin administered as a 500 mg dose, three times a day, for 1 week (n=6), reduced the clearance of midazolam following a single 0.5 mg/kg IV dose. The half-life was approximately doubled.
- The effects of diltiazem (60 mg three times a day) and verapamil (80 mg three times a day) on the pharmacokinetics and pharmacodynamics of midazolam were investigated in a three-way crossover study (n=9). The half-life of midazolam increased from 5 to 7 hours when midazolam was taken in conjunction with verapamil or diltiazem. No interaction was observed in healthy subjects between midazolam and nifedipine.
- In a placebo-controlled study, where saquinavir or placebo was administered orally as a 1200 mg dose three times a day for 5 days (n=12), a 56% reduction in the clearance of midazolam following a single 0.05 mg/kg IV dose was observed. The half-life was approximately doubled.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Midazolam maleate was administered in the diet to mice and rats for 2 years at doses of 0, 1, 9, or 80 mg/kg/day. In female mice in the highest dose group there was a marked increase in the incidence of hepatic tumors. In high-dose male rats there was a small but statistically significant increase in benign thyroid follicular cell tumors. The highest dose not associated with increased tumor incidences in mice and rats (9 mg/kg/day) is approximately 4 and 9 times, respectively, the recommended human dose (RHD) of 10 mg based on body surface area (mg/m2). The pathogenesis of induction of these tumors is not known. These tumors were found after chronic administration, whereas human use will ordinarily be of single or several doses.
Mutagenesis
Midazolam was negative for genotoxicity in in vitro (Ames, mammalian cell clastogenicity) and in vivo (mouse bone marrow micronucleus) assays.
Impairment of Fertility
When midazolam (0, 1, 4, or 16 mg/kg) was orally administered to male and female rats prior to and during mating and continuing in females throughout gestation and lactation, no adverse effects on male or female fertility were noted. Midazolam plasma exposures (AUC) at the highest dose tested were approximately 6 times that in humans at the RHD.
-
14 CLINICAL STUDIES
The safety and effectiveness of Seizalam for the treatment of status epilepticus was established in a multi-center, randomized, double-blind (double-dummy), active-control trial comparing midazolam administered intramuscularly (IM) via an auto-injector to lorazepam administered intravenously (IV). Patients meeting the diagnosis of status epilepticus, with continuing convulsive seizure activity after the arrival of paramedics, were eligible for enrollment. The ITT population consisted of 893 patients who were randomized to receive either IM midazolam (n=448) or IV lorazepam (n=445). Following randomization, each patient received study treatments administered by a healthcare professional (e.g., paramedic) prior to arrival at a hospital. According to the double-dummy design, adult patients received 10 mg IM midazolam followed by IV placebo or received IM placebo followed by 4 mg IV lorazepam. The primary efficacy endpoint was the termination of convulsive seizure activity (without the need for rescue medication) prior to arrival at the emergency department (ED) as determined by the ED attending physician. A statistically significantly higher percentage of midazolam-treated patients met the primary efficacy endpoint, as shown in Table 2.
Table 2: Primary Efficacy Analysis Results: Seizure Termination (without rescue medication) a Fischer's exact test
IM Midazolam
(n=448)IV Lorazepam
(n= 445)Treatment success (%) 73.4 63.4 p-value a 0.002 -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Seizalam (midazolam injection) is a clear, colorless to light yellow sterile solution available in multiple-dose fliptop vials containing 50 mg/10 mL (5 mg/mL).
Seizalam is supplied in the following packaging configurations:
- One vial: NDC: 11704-650-01
- Carton of 10 vials: NDC: 11704-650-10
-
17 PATIENT COUNSELING INFORMATION
Patients having seizures will likely be unresponsive or may have difficulty in comprehending counseling information.
Risks from Concomitant Use with Opioids
Inform patients and caregivers that potentially fatal additive effects may occur if Seizalam is used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
Concomitant Medications
Advise patients to inform their physician about any alcohol consumption and medicine they are now taking, especially blood pressure medication and antibiotics, including drugs they buy without a prescription. Alcohol has an increased effect when consumed with benzodiazepines; therefore, caution should be exercised regarding simultaneous ingestion of alcohol during benzodiazepine treatment [see Warnings and Precautions (5.4), Drug Interactions (7)].
Impaired Cognitive Function
Advise patients not to operate hazardous machinery or a motor vehicle until the effects of the drug, such as drowsiness, have subsided [see Warnings and Precautions (5.5)].
Pregnancy
Instruct patients to inform their physician if they are pregnant or are planning to become pregnant. Several studies have suggested an increased risk of congenital malformations associated with the use of benzodiazepine drugs. Animal studies have demonstrated an effect on early brain development and long-term cognitive effects with exposure to anesthetic and sedation drugs in the third trimester of gestation. Encourage patients to enroll in the North American Antiepileptic Drug Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Instruct patients to inform their physician if they are nursing [see Use in Specific Populations (8.2)].
Manufactured by:
Hospira, Inc.
Lake Forest, IL 60045
A Pfizer CompanyDistributed by:
Meridian Medical Technologies, Inc.
Columbia, MD 21046
A Pfizer CompanyLAB-1070-2.0
-
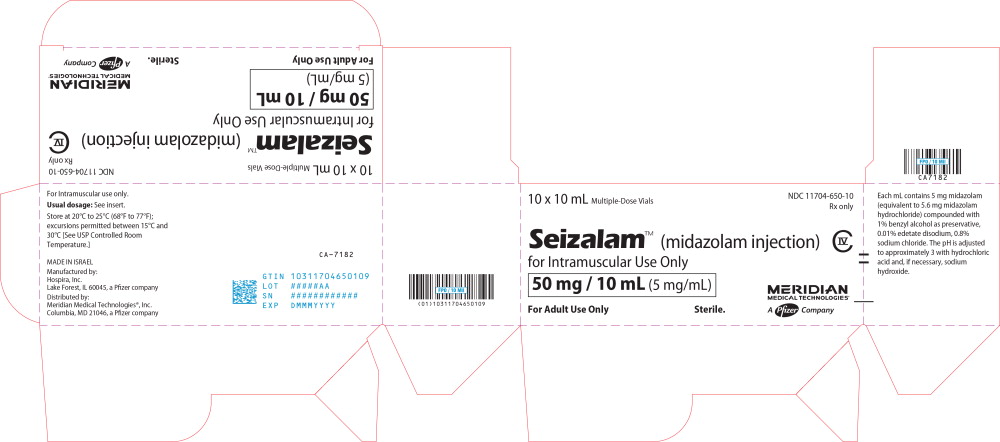
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Seizalam 5mg/mL Carton Label
10 x 10 mL Multiple-Dose Vials
NDC: 11704-650-10
Rx only
Seizalam™ (midazolam injection)
for Intramuscular Use Only50 mg / 10 mL (5 mg/mL)
For Adult Use Only
Sterile.
CIV
MERIDIAN
MEDICAL TECHNOLOGIES®A Pfizer Company
-
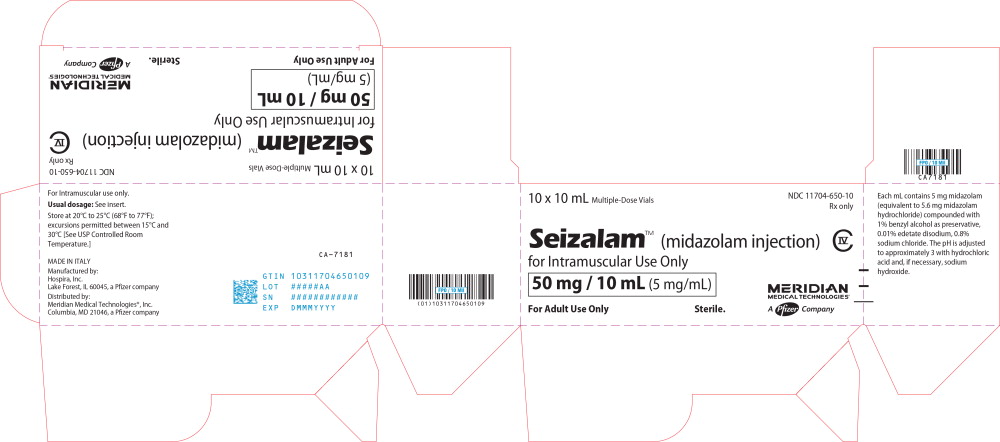
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Seizalam 5mg/mL Vial Label
Rx only
NDC: 11704-650-01
Seizalam™ CIV
(midazolam injection)
Multiple-Dose Vial for Intramuscular Use Only
50 mg / 10 mL (5 mg/mL)
For Adult Use Only
Sterile.
-
INGREDIENTS AND APPEARANCE
SEIZALAM
midazolam hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 11704-650 Route of Administration INTRAMUSCULAR DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength midazolam hydrochloride (UNII: W7TTW573JJ) (midazolam - UNII:R60L0SM5BC) midazolam 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) edetate disodium (UNII: 7FLD91C86K) benzyl alcohol (UNII: LKG8494WBH) hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11704-650-10 10 in 1 CARTON 09/14/2018 1 NDC: 11704-650-01 10 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209566 09/14/2018 Labeler - Meridian Medical Technologies, Inc. (167671341) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 030606222 ANALYSIS(11704-650) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 093132819 ANALYSIS(11704-650) , LABEL(11704-650) , MANUFACTURE(11704-650) , PACK(11704-650) Establishment Name Address ID/FEI Business Operations Hospira, Inc. 827731089 ANALYSIS(11704-650)
Trademark Results [Seizalam]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SEIZALAM 88181905 not registered Live/Pending |
Meridian Medical Technologies, Inc. 2018-11-05 |
 SEIZALAM 86429708 not registered Dead/Abandoned |
Meridian Medical Technologies, Inc. 2014-10-21 |
 SEIZALAM 85426909 not registered Dead/Abandoned |
Meridian Medical Technologies, Inc. 2011-09-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.