reduSAN by sanPharma GmbH reduSAN
reduSAN by
Drug Labeling and Warnings
reduSAN by is a Other medication manufactured, distributed, or labeled by sanPharma GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
REDUSAN- ascophyllum nodosum, pomegranate, and cocoa capsule
sanPharma GmbH
----------
reduSAN
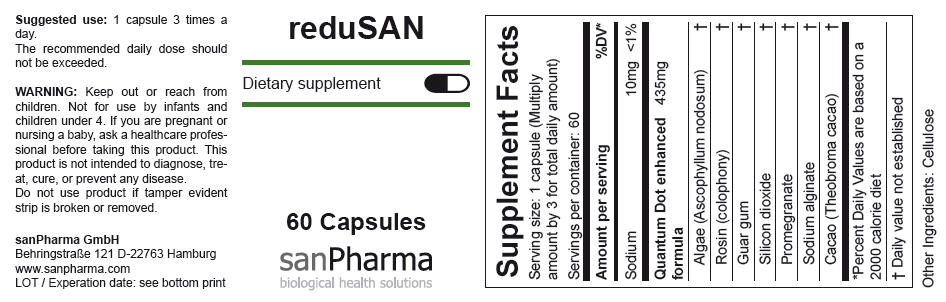
| Supplement Facts | ||
|---|---|---|
| Serving size: 1 capsule (Multiply amount by 3 for total daily amount) | ||
| Servings per container: 60 | ||
| Amount per serving | %DV* | |
|
|
||
| Sodium | 10mg | <1% |
| Quantum Dot enhanced formula | 435mg | |
| Algae (Ascophyllum nodosum) | † | |
| Rosin (colophony) | † | |
| Guar gum | † | |
| Silicon dioxide | † | |
| Promegranate | † | |
| Sodium alginate | † | |
| Cacao (Theobroma cacao) | † | |
Other Ingredients: Cellulose
WARNING
Keep out or reach from children. Not for use by infants and children under 4. If you are pregnant or nursing a baby, ask a healthcare professional before taking this product. This product is not intended to diagnose, treat, cure, or prevent any disease.
Do not use product if tamper evident strip is broken or removed.
| REDUSAN
ascophyllum nodosum, pomegranate, and cocoa capsule |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 22 mm | |
| Labeler - sanPharma GmbH (341409153) |
Revised: 10/2019
Document Id: a24c5765-b837-4526-ac68-b79c50d17d05
Set id: cb5ad340-2a36-4957-ab56-0e498285f89b
Version: 2
Effective Time: 20191015
Trademark Results [reduSAN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REDUSAN 76264370 2990837 Dead/Cancelled |
PRIMEX EHF 2001-05-29 |
 REDUSAN 74255617 not registered Dead/Abandoned |
Halsoprodukter Lars Karnerud AB 1992-03-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.