METOPROLOL TARTRATE- metoprolol tablet
METOPROLOL TARTRATE by
Drug Labeling and Warnings
METOPROLOL TARTRATE by is a Prescription medication manufactured, distributed, or labeled by Alembic Pharmaceuticals Limited, Alembic Pharmaceuticals Limited (Formulation Division-IV). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Metoprolol tartrate, USP is a selective beta1-adrenoreceptor blocking agent, available as 25 mg, 50 mg and 100 mg tablets for oral administration. Metoprolol tartrate USP is (±)-1-(Isopropylamino)-3-[p-2-methoxyethyl)phenoxy]-2-propanol L-(+)-tartrate (2:1) salt, its structural formula is:
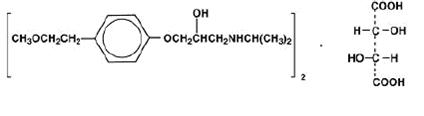
Metoprolol tartrate USP is a white, practically odorless, crystalline powder with a molecular weight of 684.82. It is very soluble in water; freely soluble in methylene chloride, in chloroform, and in alcohol; slightly soluble in acetone; and insoluble in ether.
Each tablet for oral administration contains 25 mg, 50 mg or 100 mg of metoprolol tartrate and the following inactive ingredients: microcrystalline cellulose, lactose monohydrate, povidone, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate hypromellose, titanium dioxide and polyethylene glycol. In addition, the 50 mg product contains D&C Red No. 30 Aluminum Lake and the 100 mg product contains FD&C Blue No. 2 Aluminum Lake and FD&C Blue No. 1 Aluminum Lake as coloring agents.
-
CLINICAL PHARMACOLOGY
Mechanism of Action:
Metoprolol tartrate is a beta1- selective (cardioselective) adrenergic receptor blocker. This preferential effect is not absolute, however, and at higher plasma concentrations, metoprolol also inhibits beta2- adrenoreceptors, chiefly located in the bronchial and vascular musculature.
Clinical pharmacology studies have confirmed the beta-blocking activity of metoprolol in man, as shown by (1) reduction in heart rate and cardiac output at rest and upon exercise, (2) reduction of systolic blood pressure upon exercise, (3) inhibition of isoproterenol-induced tachycardia, and (4) reduction of reflex orthostatic tachycardia.
Hypertension
The mechanism of the antihypertensive effects of beta-blocking agents has not been fully elucidated.However, several possible mechanisms have been proposed: (1) competitive antagonism of catecholamines at peripheral (especially cardiac) adrenergic neuron sites, leadingto decreased cardiac output; (2) a central effect leading to reduced sympathetic outflow to the periphery; and (3) suppression of renin activity.
Angina Pectoris
By blocking catecholamine-induced increases in heart rate, in velocity and extent of myocardial contraction, and in blood pressure, metoprolol reduces the oxygen requirements of the heart at any given level of effort, thus making it useful in the long-term management of angina pectoris.
Myocardial Infarction
The precise mechanism of action of metolrolol in patients with suspected or definite myocardial infarction is not known.
Pharmacodynamics
Relative beta1 selectivity is demonstrated by the following: (1) In healthy subjects, metoprolol is unable to reverse the beta2-mediated vasodilating effects of epinephrine. This contrasts with the effect of nonselective (beta1 plus beta2) beta blockers, which completely reverse the vasodilating effects of epinephrine. (2) In asthmatic patients, metoprolol reduces FEV1 and FVC significantly less than a nonselective beta-blocker, propranolol, at equivalent beta1-receptor blocking doses.
Metoprolol has no intrinsic sympathomimetic activity, and membrane-stabilizing activity is detectable only at doses much greater than required for beta-blockade. Animal and human experiments indicate that metoprolol slows the sinus rate and decreases AV nodal conduction.
Significant beta-blocking effect (as measured by reduction of exercise heart rate) occurs within 1 hour after oral administration, and its duration is dose-related. For example, a 50% reduction of the maximum effect after single oral doses of 20, 50, and 100 mg occurred at 3.3, 5, and 6.4 hours, respectively, in normal subjects. After repeated oral dosages of 100 mg twice daily, a significant reduction in exercise systolic blood pressure was evident at 12 hours. When the drug was infused over a 10-minute period, in normal volunteers, maximum beta blockade was achieved at approximately 20 minutes. Equivalent maximal beta-blocking effect is achieved with oral and intravenous doses in the ratio of approximately 2.5:1.
There is a linear relationship between the log of plasma levels and reduction of exercise heart rate. However, antihypertensive activity does not appear to be related to plasma levels. Because of variable plasma levels attained with a given dose and lack of a consistent relationship of antihypertensive activity to dose, selection of proper dosage requires individual titration.
In several studies of patients with acute myocardial infarction, intravenous followed by oral administration of metoprolol caused a reduction in heart rate, systolic blood pressure and cardiac output. Stroke volume, diastolic blood pressure and pulmonary artery end diastolic pressure remained unchanged.
In patients with angina pectoris, plasma concentration measured at 1 hour is linearly related to the oral dose within the range of 50 to 400 mg. Exercise heart rate and systolic blood pressure are reduced in relation to the logarithm of the oral dose of metoprolol. The increase in exercise capacity and the reduction in left ventricular ischemia are also significantly related to the logarithm of the oral dose.
Pharmacokinetics
Absorption: The estimated oral bioavailability of immediate release metoprolol is about 50% because of pre-systemic metabolism which is saturable leading to non-proportionate increase in the exposure with increased dose.
Distribution: Metoprolol is extensively distributed with a reported volume of distribution of 3.2 to 5.6 L/kg. About 10% of metoprolol in plasma is bound to serum albumin. Metoprolol is known to cross the placenta and is found in breast milk. Metoprolol is also known to cross the blood brain barrier following oral administration and CSF concentrations close to that observed in plasma have been reported. Metoprolol is not a significant P-glycoprotein substrate
Metabolism: Metoprolol is primarily metabolized by CYP2D6. Metoprolol is a racemic mixture of R- and S- enantiomers, and when administered orally, it exhibits stereoselective metabolism that is dependent on oxidation phenotype. CYP2D6 is absent (poor metabolizers) in about 8% of Caucasians and about 2% of most other populations. Poor CYP2D6 metabolizers exhibit several-fold higher plasma concentrations of metoprolol than extensive metabolizers with normal CYP2D6 activity thereby decreasing metoprolol’s cardioselectivity.
Elimination: Elimination of metoprolol is mainly by biotransformation in the liver. The mean elimination half-life of metoprolol is 3 to 4 hours; in poor CYP2D6 metabolizers the half-life may be 7 to 9 hours. Approximately 95% of the dose can be recovered in urine. In most subjects (extensive metabolizers), less than 5% of an oral dose are excreted as unchanged drug in the urine. In poor metabolizers, up to 30% of oral doses,may be excreted unchanged; the rest is excreted by the kidneys as metabolites that appear to have no beta blocking activity. The renal clearance of the stereo-isomers does not exhibit stereo-selectivity in renal excretion.
Special populations
Geriatric patients: The geriatric population may show slightly higher plasma concentrations of metoprolol as a combined result of a decreased metabolism of the drug in elderly population and a decreased hepatic blood flow. However, this increase is not clinically significant or therapeutically relevant.
Renal impairment: The systemic availability and half-life of metoprolol in patients with renal failure do not differ to a clinically significant degree from those in normal subjects.
Hepatic Impairment: Since the drug is primarily eliminated by hepatic metabolism, hepatic impairment may impact the pharmacokinetics of metoprolol. The elimination half-life of metoprolol is considerably prolonged, depending on severity (up to 7.2 h).
Clinical Studies:
Hypertension
In controlled clinical studies, metoprolol has been shown to be an effective antihypertensive agent when used alone or as concomitant therapy with thiazide-type diuretics, at dosages of 100 to 450 mg daily. In controlled, comparative, clinical studies, metoprolol has been shown to be as effective an antihypertensive agent as propranolol, methyldopa, and thiazide-type diuretics, to be equally effective in supine and standing positions.
Angina Pectoris
In controlled clinical trials, metoprolol, administered two or four times daily, has been shown to be an effective antianginal agent, reducing the number of angina attacks and increasing exercise tolerance. The dosage used in these studies ranged from 100 to 400 mg daily. A controlled, comparative, clinical trial showed that metoprolol was indistinguishable from propranolol in the treatment of angina pectoris.
Myocardial Infarction
In a large (1,395 patients randomized), double-blind, placebo-controlled clinical study, metoprolol was shown to reduce 3-month mortality by 36% in patients with suspected or definite myocardial infarction.
Patients were randomized and treated as soon as possible after their arrival in the hospital, once their clinical condition had stabilized and their hemodynamic status had been carefully evaluated. Subjects were ineligible if they had hypotension, bradycardia, peripheral signs of shock, and/or more than minimal basal rales as signs of congestive heart failure. Initial treatment consisted of intravenous followed by oral administration of metoprolol or placebo, given in a coronary care or comparable unit. Oral maintenance therapy with metoprolol or placebo was then continued for 3 months. After this double-blind period, all patients were given metoprolol and followed up to 1 year.
The median delay from the onset of symptoms to the initiation of therapy was 8 hours in both the metoprolol- and placebo-treatment groups. Among patients treated with metoprolol, there were comparable reductions in 3-month mortality for those treated early (≤8 hours) and those in whom treatment was started later. Significant reductions in the incidence of ventricular fibrillation and in chest pain following initial intravenous therapy were also observed with metoprolol and were independent of the interval between onset of symptoms and initiation of therapy.
In this study, patients treated with metoprolol received the drug both very early (intra-venously) and during a subsequent 3-month period, while placebo patients received no beta-blocker treatment for this period. The study thus was able to show a benefit from the overall metoprolol regimen but cannot separate the benefit of very early intravenous treatment from the benefit of later beta-blocker therapy. Nonetheless, because the overall regimen showed a clear beneficial effect on survival without evidence of an early adverse effect on survival, one acceptable dosage regimen is the precise regimen used in the trial. Because the specific benefit of very early treatment remains to be defined however, it is also reasonable to administer the drug orally to patients at a later time as is recommended for certain other beta- blockers.
-
INDICATIONS & USAGE
Hypertension
Metoprolol tartrate tablets are indicated for the treatment of hypertension. They may be used alone or in combination with other antihypertensive agents.
Angina Pectoris
Metoprolol tartrate tablets are indicated in the long-term treatment of angina pectoris.
Myocardial Infarction
Metoprolol tartrate tablets are indicated in the treatment of hemodynamically stable patients with definite or suspected acute myocardial infarction to reduce cardiovascular mortality when used alone or in conjunction with intravenous metoprolol. Oral metoprolol therapy can be initiated after intravenous metoprolol therapy or, alternatively, oral treatment can begin within 3 to 10 days of the acute event (see DOSAGE AND ADMINISTRATION, CONTRAINDICATIONS, and WARNINGS.
-
CONTRAINDICATIONS
Hypertension and Angina
Metoprolol tartrate tablets are contraindicated in sinus bradycardia, heart block greater than first degree, cardiogenic shock, and overt cardiac failure (see WARNINGS).
Hypersensitivity to metoprolol and related derivatives, or to any of the excipients; hypersensitivity to other beta-blockers (crosssensitivity between beta-blockers can occur).
Sick-sinus syndrome.
Severe peripheral arterial circulatory disorders.
Myocardial Infarction
Metoprolol is contraindicated in patients with a heart rate < 45 beats/min; second- and third-degree heart block; significant first-degree heart block (P-R interval ≥ 0.24 sec); systolic blood pressure < 100 mmHg; or moderate to severe cardiac failure (see WARNINGS). -
WARNINGS
Heart Failure
Beta blockers, like metoprolol, can cause depression of myocardial contractility and may precipitate heart failure and cardiogenic shock. If signs or symptoms of heart failure develop, treat the patient according to recommended guidelines. It may be necessary to lower the dose of metoprolol or to discontinue it.Ischemic Heart Disease
Do not abruptly discontinue metoprolol therapy in patients with coronary artery disease. Severe exacerbation of angina, myocardial infarction and ventricular arrhythmias have been reported in patients with coronary artery disease following the abrupt discontinuation of therapy with beta-blockers.When discontinuing chronically administered metoprolol, particularly in patients with coronary artery disease, the dosage should be gradually reduced over a period of 1 to 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, metoprolol administration should be reinstated promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physicians advice.
Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue metoprolol therapy abruptly even in patients treated only for hypertension.
Use During Major Surgery
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.Bradycardia
Bradycardia, including sinus pause, heart block, and cardiac arrest have occurred with the use of metoprolol. Patients with first-degree atrioventricular block, sinus node dysfunction, or conduction disorders may be at increased risk. Monitor heart rate and rhythm in patients receiving metoprolol. If severe bradycardia develops, reduce or stop metoprolol.
Exacerbation of Bronchospastic Diseases
Patients with bronchospastic diseases, should, in general, not receive beta blockers, including metoprolol. Because of its relative beta1 selectivity, however, metoprolol may be used in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Because beta1 selectivity is not absolute use, the lowest possible dose of metoprolol tartrate and consider administering metoprolol in smaller doses three times daily, instead of larger doses two times daily, to avoid the higher plasma levels associated with the longer dosing interval (see DOSAGE AND ADMINISTRATION). Bronchodilators, including beta2 agonists, should be readily available or administered concomitantly.Diabetes and Hypoglycemia
Beta-blockers may mask tachycardia occurring with hypoglycemia, but other manifestations such as dizziness and sweating may not be significantly affected.
Pheochromocytoma
If metoprolol is used in the setting of pheochromocytoma, it should be given in combination with an alpha-blocker, and only after the alpha-blocker has been initiated. Administration of beta-blockers alone in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure due to the attenuation of beta-mediated vasodilatation in skeletal muscle.
Thyrotoxicosis
Metoprolol tartrate tablets may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Avoid abrupt withdrawal of beta-blockade, which might precipitate a thyroid storm.
-
PRECAUTIONS
Risk of Anaphylactic Reactions
While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
Information for patients
Advise patients to take metoprolol regularly and continuously, as directed, with or immediately following meals. If a dose should be missed, the patient should take only the next scheduled dose (without doubling it). Patients should not discontinue metoprolol without consulting the physician.
Advise patients (1) to avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient’s response to therapy with metoprolol has been determined; (2) to contact the physician if any difficulty in breathing occurs; (3) to inform the physician or dentist before any type of surgery that he or she is taking metoprolol.
DRUG INTERACTIONS
Catecholamine-depleting drugs: Catecholamine-depleting drugs (e.g., reserpine) may have an additive effect when given with beta-blocking agents or monoamine oxidase (MAO) inhibitors. Observe patients treated with metoprolol plus a catecholamine depletor for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or
postural hypotension.
In addition, possibly significant hypertension may theoretically occur up to 14 days following discontinuation of the concomitant administration with an irreversible MAO inhibitor.
Digitalis glycosides and beta blockers:Both digitalis glycosides and beta blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia. Monitor heart rate and PR interval.
Calcium channel blockers:Concomitant administration of a beta-adrenergic antagonist with a calcium channel blocker may produce an additive reduction in myocardial contractility because of negative chronotropic and inotropic effects.
CYP2D6 Inhibitors:Potent inhibitors of the CYP2D6 enzyme may increase the plasma concentration of metoprolol which would mimic the pharmacokinetics of CYP2D6 poormetabolizer (see Pharmacokinetics section). Increase in plasma concentrations of metoprolol would decrease the cardioselectivity of metoprolol. Known clinically significant potent inhibitors of CYP2D6 are antidepressants such as fluvoxamine, fluoxetine, paroxetine, sertraline, bupropion, clomipramine, and desipramine; antipsychotics such as chlorpromazine, fluphenazine, haloperidol, and thioridazine; antiarrhythmics such as quinidine or propafenone; antiretrovirals such as ritonavir; antihistamines such as diphenhydramine; antimalarials such as hydroxychloroquine or quinidine; antifungals such as terbinafine.
Hydralazine:Concomitant administration of hydralazine may inhibit presystemic metabolism of metoprolol leading to increased concentrations of metoprolol.
Alpha-adrenergic agents:Antihypertensive effect of alpha-adrenergic blockers such as guanethidine, betanidine, reserpine, alpha-methyldopa or clonidine may be potentiated by beta-blockers including metoprolol. Beta-adrenergic blockers may also potentiate the postural hypotensive effect of the first dose of prazosin, probably by preventing reflex tachycardia. On the contrary, beta adrenergic blockers may also potentiate the hypertensive response to withdrawal of clonidine in patients receiving concomitant clonidine and beta-adrenergic blocker. If a patient is treated with clonidine and metoprolol concurrently, and clonidine treatment is to be discontinued, stop metoprolol several days before clonidine is withdrawn. Rebound hypertension that can follow withdrawal of clonidine may be increased in patients receiving concurrent beta-blocker treatment.
Ergot alkaloid:Concomitant administration with beta-blockers may enhance the vasoconstrictive action of ergot alkaloids.
Dipyridamole:In general, administration of a beta-blocker should be withheld before dipyridamole testing, with careful monitoring of heart rate following the dipyridamole injection.
Carcinogenesis & Mutagenesis & Impairment Of Fertility
Long-term studies in animals have been conducted to evaluate carcinogenic potential. In a 2-year study in rats at three oral dosage levels of up to 800 mg/kg per day, there was no increase in the development of spontaneously occurring benign or malignant neoplasms of any type. The only histologic changes that appeared to be drug related were an increased incidence of generally mild focal accumulation of foamy macrophages in pulmonary alveoli and a slight increase in biliary hyperplasia. In a 21-month study in Swiss albino mice at three oral dosage levels of up to 750 mg/kg per day, benign lung tumors (small adenomas) occurred more frequently in female mice receiving the highest dose than in untreated control animals. There was no increase in malignant or total (benign plus malignant) lung tumors, or in the overall incidence of tumors or malignant tumors. This 21-month study was repeated in CD-1 mice, and no statistically or biologically significant differences were observed between treated and control mice of either sex for any type of tumor.
All mutagenicity tests performed (a dominant lethal study in mice, chromosome studies in somatic cells, a Salmonella/mammalian-microsome mutagenicity test, and a nucleus anomaly test in somatic interphase nuclei) were negative.
Reproduction toxicity studies in mice, rats and rabbits did not indicate teratogenic potential for metoprolol tartrate. Embryotoxicity and/or fetotoxicity in rats and rabbits were noted starting at doses of 50 mg/kg in rats and 25 mg/kg in rabbits, as demonstrated by increases in preimplantation loss, decreases in the number of viable fetuses per dose, and/or decreases in neonatal survival. High doses were associated with some maternal toxicity, and growth delay of the offspring in utero, which was reflected in minimally lower weights at birth. The oral NOAELs for embryo-fetal development in mice, rats, and rabbits were considered to be 25, 200, and 12.5 mg/kg. This corresponds to dose levels that are approximately 0.3, 4, and 0.5 times, respectively, when based on surface area, the maximum human oral dose (8 mg/kg/day) of metoprolol tartrate. Metoprolol tartrate has been associated with reversible adverse effects on spermatogenesis starting at oral dose levels of 3.5 mg/kg in rats (a dose that is only 0.1-times the human dose, when based on surface area), although other studies have shown no effect of metoprolol tartrate on reproductive performance in male rats.
Pregnancy Category C
Upon confirming the diagnosis of pregnancy, women should immediately inform the doctor.
Metoprolol has been shown to increase postimplantation loss and decrease neonatal survival in rats at doses up to 11 times the maximum daily human dose of 450 mg, when based on surface area. Distribution studies in mice confirm exposure of the fetus when metoprolol is administered to the pregnant animal. These limited animal studies do not indicate direct or indirect harmful effects with respect to teratogenicity (see Carcinogenesis, Mutagenesis, Impairment of Fertility). There are no adequate and well-controlled studies in pregnant women. The amount of data on the use of metoprolol in pregnant women is limited. The risk to the fetus/mother is unknown. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Metoprolol is excreted in breast milk in a very small quantity. An infant consuming one liter of breast milk daily would receive a dose of less than 1 mg of the drug.
Fertility
The effects of Metoprolol on the fertility of human have not been studied.
Metoprolol showed effects on spermatogenesis in male rats at a therapeutic dose level, but had no effect on rates of conception at higher doses in animal fertility studies (see Carcinogenesis, Mutagenesis, Impairment of Fertility).
Geriatric Use
Clinical trials of metoprolol in hypertension did not include sufficient numbers of elderly patients to determine whether patients over 65 years of age differ from younger subjects in their response to metoprolol. Other reported clinical experience in elderly hypertensive patients has not identified any difference in response from younger patients.
In worldwide clinical trials of metoprolol in myocardial infarction, where approximately 478 patients were over 65 years of age (0 over 75 years of age), no age-related differences in safety and effectiveness were found. Other reported clinical experience in myocardial infarction has not identified differences in response between the elderly and younger patients. However, greater sensitivity of some elderly individuals taking metoprolol cannot be categorically ruled out. Therefore, in general, it is recommended that dosing proceed with caution in this population.
-
ADVERSE REACTIONS
Hypertension and Angina
Most adverse effects have been mild and transient.
Central Nervous System: Tiredness and dizziness have occurred in about 10 of 100 patients. Depression has been reported in about 5 of 100 patients. Mental confusion and short-term memory loss have been reported. Headache, nightmares, and insomnia have also been reported.
Cardiovascular: Shortness of breath and bradycardia have occurred in approximately 3 of 100 patients. Cold extremities; arterial insufficiency, usually of the Raynaud type; palpitations; congestive heart failure; peripheral edema; and hypotension have been reported in about 1 of 100 patients. Gangrene in patients with pre-existing severe peripheral circulatory disorders has also been reported very rarely (see CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS).
Respiratory: Wheezing (bronchospasm) and dyspnea have been reported in about 1 of 100 patients (see WARNINGS). Rhinitis has also been reported.
Gastrointestinal: Diarrhea has occurred in about 5 of 100 patients. Nausea, dry mouth, gastric pain, constipation, flatulence, and heartburn have been reported in about 1 of 100 patients. Vomiting was a common occurrence. Post-marketing experience reveals very rare reports of hepatitis, jaundice and nonspecific hepatic dysfunction. Isolated cases of transaminase, alkaline phosphatase, and lactic dehydrogenase elevations have also been reported.
Hypersensitive Reactions: Pruritus or rash have occurred in about 5 of 100 patients. Very rarely, photosensitivity and worsening of psoriasis have been reported.
Miscellaneous:
Peyronie’s disease has been reported in fewer than 1 of 100,000 patients. Musculoskeletal pain, blurred vision, and tinnitus have also been reported.
There have been rare reports of reversible alopecia, agranulocytosis, and dry eyes. Discontinuation of the drug should be considered if any such reaction is not otherwise explicable. There have been very rare reports of weight gain, arthritis, and retroperitoneal fibrosis (relationship to metoprolol has not been definitely established).
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with metoprolol.
Myocardial Infarction
Central Nervous System: Tiredness has been reported in about 1 of 100 patients. Vertigo, sleep disturbances, hallucinations, headache, dizziness, visual disturbances, confusion, and reduced libido have also been reported, but a drug relationship is not clear.
Cardiovascular: In the randomized comparison of metoprolol and placebo described in the CLINICAL PHARMACOLOGY section, the following adverse reactions were reported:
Metoprolol
Placebo
Hypotension (systolic BP <90 mm Hg)
27.4%
23.2%
Bradycardia (heart rate <40 beats/min)
15.9%
6.7%
Second- or third-degree heart block
4.7%
4.7%
First-degree heart block (P-R ≥0.26 sec)
5.3%
1.9%
Heart failure
27.5%
29.6%
Respiratory: Dyspnea of pulmonary origin has been reported in fewer than 1 of 100 patients.
Gastrointestinal: Nausea and abdominal pain have been reported in fewer than 1 of 100 patients.
Dermatologic: Rash and worsened psoriasis have been reported, but a drug relationship is not clear.
Miscellaneous: Unstable diabetes and claudication have been reported, but a drug relationship is not clear.
Potential Adverse Reactions
A variety of adverse reactions not listed above has been reported with other beta-adrenergic blocking agents and should be considered potential adverse reactions to metoprolol.
Central Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics.
Cardiovascular: Intensification of AV block (see CONTRAINDICATIONS).
Hematologic: Agranulocytosis, nonthrombocytopenic purpura and thrombocytopenic purpura.
Hypersensitive Reactions: Fever combined with aching and sore throat, laryngospasm and respiratory distress.
Post-Marketing Experience
The following adverse reactions have been reported during post-approval use of metoprolol tartrate: confusional state, an increase in blood triglycerides and a decrease in High Density Lipoprotein (HDL). Because these reports are from a population of uncertain size and are subject to confounding factors, it is not possible to reliably estimate their frequency.
-
OVERDOSAGE
Acute Toxicity
Several cases of overdosage have been reported, some leading to death.
Oral LD50’s (mg/kg): mice, 1,158 to 2,460; rats, 3,090 to 4,670.
Signs and Symptoms
Potential signs and symptoms associated with overdosage with metoprolol are bradycardia, hypotension, bronchospasm, myocardial infarction, cardiac failure and death.
Management
There is no specific antidote.
In general, patients with acute or recent myocardial infarction may be more hemodynamically unstable than other patients and should be treated accordingly (see WARNINGS: Myocardial Infarction).
On the basis of the pharmacologic actions of metoprolol, the following general measures should be employed:
Elimination of the Drug:Gastric lavage should be performed.
Other clinical manifestations of overdose should be managed symptomatically based on modern methods of intensive care.
Hypotension:Administer a vasopressor, e.g.,levarterenol or dopamine.
Bronchospasm:Administer a beta2-stimulating agent and/or a theophylline derivative.
Cardiac Failure:
Administer digitalis glycoside and diuretic. In shock resulting from inadequate cardiac contractility, consider administration of dobutamine, isoproterenol or glucagon.
-
DOSAGE & ADMINISTRATION
Hypertension
Individualize the dosage of metoprolol tablets. Metoprolol tartrate tablets should be taken with or immediately following meals.
The usual initial dosage of metoprolol tartrate tablets is 100 mg daily in single or divided doses, whether used alone or added to a diuretic. Increase the dosage at weekly (or longer) intervals until optimum blood pressure reduction is achieved. In general, the maximum effect of any given dosage level will be apparent after one week of therapy. The effective dosage range of metoprolol tartrate tablets is 100 to 450 mg per day. Dosages above 450 mg per day have not been studied. While once daily dosing is effective and can maintain a reduction in blood pressure throughout the day, lower doses (especially 100 mg) may not maintain a full effect at the end of the 24-hour period, and larger or more frequent daily doses may be required. This can be evaluated by measuring blood pressure near the end of the dosing interval to determine whether satisfactory control is being maintained throughout the day. Beta1 selectivity diminishes as the dose of metoprolol is increased.
Angina Pectoris
The dosage of metoprolol tartrate tablets should be individualized. Metoprolol tartrate tablets should be taken with or immediately following meals.
The usual initial dosage of metoprolol tartrate tablets is 100 mg daily, given in two divided doses. gradually increased at weekly intervals until optimum clinical response has been obtained or there is pronounced slowing of the heart rate.
The effective dosage range of metoprolol tartrate tablets is 100 to 400 mg per day. Dosages above 400 mg per day have not been studied. If treatment is to be discontinued, gradually decrease the dosage over a period of 1 to 2 weeks (see WARNINGS).
Myocardial Infarction
Early Treatment:During the early phase of definite or suspected acute myocardial infarction, treatment with metoprolol tartrate tablets can be initiated as soon as possible after the patient’s arrival in the hospital. Such treatment should be initiated in a coronary care or similar unit immediately after the patient’s hemodynamic condition has stabilized.
Begin treatment in this early phase should begin with the intravenous administration of three bolus injections of 5 mg of metoprolol tartrate each; the injections should be given at approximately 2 minute intervals. During the intravenous administration of metoprolol, monitor blood pressure, heart rate, and electrocardiogram.
In patients who tolerate the full intravenous dose (15 mg), initiate metoprolol tartrate tablets, 50 mg every 6 hours, 15 minutes after the last intravenous dose and continue for 48 hours. Thereafter, the maintenance dosage is 100 mg twice daily (see Late Treatment below).
Start patients who appear not to tolerate the full intravenous dose on metoprolol tablets either 25 mg or 50 mg every 6 hours (depending on the degree of intolerance) 15 minutes after the last intravenous dose or as soon as their clinical condition allows. In patients with severe intolerance, discontinue metoprolol(see WARNINGS).
Late Treatment:Start patients with contraindications to treatment during the early phase of suspected or definite myocardial infarction, patients who appear not to tolerate the full early treatment, and patients in whom the physician wishes to delay therapy for any other reason on metoprolol tartrate tablets, 100 mg twice daily, as soon as their clinical condition allows. Continue therapy for at least 3 months. Although the efficacy of metoprolol beyond 3 months has not been conclusively established, data from studies with other beta-blockers suggest that treatment should be continued for 1 to 3 years.
Special populations
Pediatric patients:No pediatric studies have been performed. The safety and efficacy of metoprolol in pediatric patients have not been established.
Renal impairment:No dose adjustment of metoprolol is required in patients with renal impairment.
Hepatic impairment:Metoprolol blood levels are likely to increase substantially in patients with hepatic impairment. Therefore, metoprolol should be initiated at low doses with cautious gradual dose titration according to clinical response.
Geriatric patients (>65 years):In general, use a low initial starting dose in elderly patients given their greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Method of administration:
For oral treatment, the tablets should be swallowed un-chewed with a glass of water. Metoprolol tartrate tablets should always be taken in standardized relation with meals. If the physician asks the patient to take metoprolol tartrate tablets either before breakfast or with breakfast, then the patient should continue taking metoprolol tartrate tablets with the same schedule during the course of therapy.
-
HOW SUPPLIED
Metoprolol Tartrate Tablets, USP are available containing 25 mg, 50 mg or 100 mg of metoprolol tartrate, USP.
The 25 mg tablets are white to off white film coated, round, biconvex tablets debossed with ‘L150’ on one side and breakline on other side. They are available as follows:
NDC: 46708-290-30 bottles of 30 tablets
NDC 46708-290-31 bottles of 100 tablets
NDC 46708-290-71 bottles of 500 tablets
NDC 46708-290-91 bottles of 1000 tablets
NDC 46708-290-10 100 (10 x 10) Tablets Unit-dose blisters
The 50 mg tablets are pink, film coated, round, biconvex tablets debossed with ‘L151’ on one side and breakline on other side. They are available as follows:
NDC 46708-291-30 bottles of 30 tablets
NDC 46708-291-31 bottles of 100 tablets
NDC 46708-291-91 bottles of 1000 tablets
NDC 46708-291-10 100 (10 x 10) Tablets Unit-dose blisters
The 100 mg tablets are blue, film coated, round, biconvex tablets debossed with ‘L152’ on one side and breakline on other side. They are available as follows:
NDC 46708-292-30 bottles of 30 tablets
NDC 46708-292-31 bottles of 100 tablets
NDC: 46708-292-91 bottles of 1000 tablets
NDC: 46708-292-10 100 (10 x 10) Tablets Unit-dose blisters
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Manufactured by:Alembic Pharmaceuticals Limited (Formulation Division),
Village Panelav, P. O. Tajpura, Near Baska,
Taluka-Halol, Panchmahal, Gujarat, India.
Revised: 06/2013 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 25 mg
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 50 mg
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 100 mg
-
INGREDIENTS AND APPEARANCE
METOPROLOL TARTRATE
metoprolol tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 46708-290 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOPROLOL TARTRATE (UNII: W5S57Y3A5L) (METOPROLOL - UNII:GEB06NHM23) METOPROLOL TARTRATE 25 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE K30 (UNII: U725QWY32X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) Product Characteristics Color WHITE (WHITE TO OFF WHITE) Score 2 pieces Shape ROUND Size 6mm Flavor Imprint Code L;150 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46708-290-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2013 2 NDC: 46708-290-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2013 3 NDC: 46708-290-91 1000 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2013 4 NDC: 46708-290-10 100 in 1 CARTON; Type 0: Not a Combination Product 07/22/2013 5 NDC: 46708-290-71 500 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202871 07/22/2013 METOPROLOL TARTRATE
metoprolol tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 46708-291 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOPROLOL TARTRATE (UNII: W5S57Y3A5L) (METOPROLOL - UNII:GEB06NHM23) METOPROLOL TARTRATE 50 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE K30 (UNII: U725QWY32X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) D&C RED NO. 30 (UNII: 2S42T2808B) Product Characteristics Color PINK Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code L;151 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46708-291-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2013 2 NDC: 46708-291-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2013 3 NDC: 46708-291-91 1000 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2013 4 NDC: 46708-291-10 100 in 1 CARTON; Type 0: Not a Combination Product 07/22/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202871 07/22/2013 METOPROLOL TARTRATE
metoprolol tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 46708-292 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOPROLOL TARTRATE (UNII: W5S57Y3A5L) (METOPROLOL - UNII:GEB06NHM23) METOPROLOL TARTRATE 100 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POVIDONE K30 (UNII: U725QWY32X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color BLUE Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code L;152 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46708-292-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2013 2 NDC: 46708-292-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2013 3 NDC: 46708-292-91 1000 in 1 BOTTLE; Type 0: Not a Combination Product 07/22/2013 4 NDC: 46708-292-10 100 in 1 CARTON; Type 0: Not a Combination Product 07/22/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202871 07/22/2013 Labeler - Alembic Pharmaceuticals Limited (650574663) Establishment Name Address ID/FEI Business Operations Alembic Pharmaceuticals Limited 650574671 MANUFACTURE(46708-290, 46708-291, 46708-292)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


