GAVIS-CARE ANTACID- aluminum hydroxide and magnesium carbonate suspension
GAVIS-CARE ANTACID by
Drug Labeling and Warnings
GAVIS-CARE ANTACID by is a Otc medication manufactured, distributed, or labeled by Geri-Care Pharmaceuticals, Corp, GCP Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
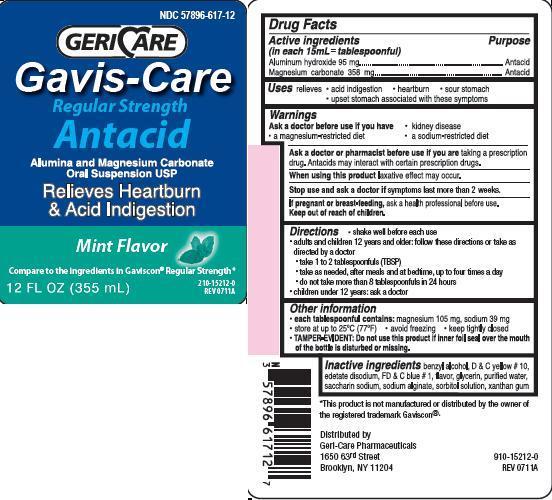
- Active ingredients (in each 15 mL = tablespoonful)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease
a magnesium-restricted diet
a sodium-restricted diet
Ask a doctor or pharmacist before use if you are taking a prescription drug.
Antacids may interact with certain prescription drugs.When using this product laxative effect may occur.
Stop use and ask a doctor if symptoms last more than 2 weeks
If pregnant or breast-feeding, ask a health professional before use.
-
Directions
shake well before each use
adults and children 12 years and older: follow these directions or take as directed by a doctor
take 1 to 2 tablespoonfuls (TBSP)
take as needed, after meals and at bedtime, up to four times a day
do not take more than 8 tablespoonfuls in 24 hours
children under 12 years: ask a doctor
- Other information
- Inactive ingredients
-
package Label
NDC: 57896-617-12
GERICARE
Gavis-CareRegular Strength
Antacid
Alumina and Magnesium Carbonate Oral Suspension USP
Relives Heartburn and Acid Indigestion
mint flavor
compare to the ingredients in Gaviscon Regular Strength
12 FL OZ (355 mL)
-
INGREDIENTS AND APPEARANCE
GAVIS-CARE ANTACID
aluminum hydroxide and magnesium carbonate suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 57896-617 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 95 mg in 15 mL MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 358 mg in 15 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM ALGINATE (UNII: C269C4G2ZQ) SORBITOL (UNII: 506T60A25R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color green (light green) Score Shape Size Flavor SPEARMINT (spearmint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57896-617-12 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/01/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 01/01/2000 Labeler - Geri-Care Pharmaceuticals, Corp (611196254) Registrant - GCP Laboratories (965480861) Establishment Name Address ID/FEI Business Operations GCP Laboratories 965480861 manufacture(57896-617)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.