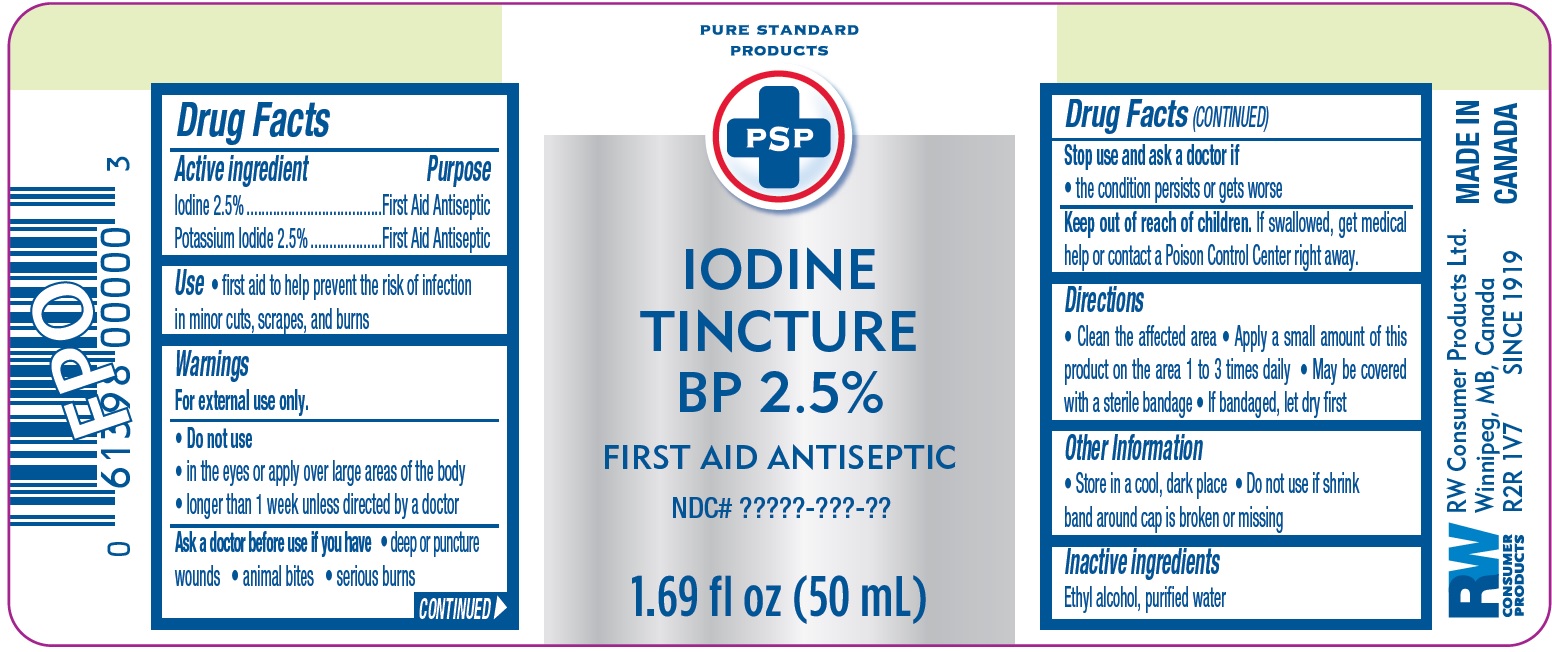
PSP Iodine tincture BP 2.5% first aid antiseptic
PSP Iodine tincture BP 2.5 first aid antiseptic by
Drug Labeling and Warnings
PSP Iodine tincture BP 2.5 first aid antiseptic by is a Otc medication manufactured, distributed, or labeled by RW Consumer Products Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PSP IODINE TINCTURE BP 2.5 FIRST AID ANTISEPTIC- iodine, potassium iodide solution
RW Consumer Products Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
PSP Iodine tincture BP 2.5% first aid antiseptic
Warnings
For external use only.
Directions
Clean the affected area Apply a small amount of this product on the area 1 to 3 times daily May be covered with a sterile bandage If bandaged, let dry first
| PSP IODINE TINCTURE BP 2.5 FIRST AID ANTISEPTIC
iodine, potassium iodide solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - RW Consumer Products Ltd (245592118) |
Revised: 6/2022
Document Id: e0e17b29-8567-e02b-e053-2995a90a3492
Set id: cba71f0f-b3f9-60e6-e053-2995a90a86cf
Version: 2
Effective Time: 20220607