ISENTRESS- raltegravir tablet, film coated
ISENTRESS by
Drug Labeling and Warnings
ISENTRESS by is a Prescription medication manufactured, distributed, or labeled by State of Florida DOH Central Pharmacy. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ISENTRESS safely and effectively. See full prescribing information for ISENTRESS.
ISENTRESS (raltegravir) Tablets
Initial U.S. Approval: 2007RECENT MAJOR CHANGES
INDICATIONS AND USAGE
ISENTRESS® is a human immunodeficiency virus integrase strand transfer inhibitor (HIV-1 INSTI) indicated:
- In combination with other antiretroviral agents for the treatment of HIV-1 infection in adult patients (1).
The safety and efficacy of ISENTRESS have not been established in pediatric patients (1).
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 400 mg (3).
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
Monitor for Immune Reconstitution Syndrome (5.1).
ADVERSE REACTIONS
- The most common adverse reactions of moderate to severe intensity (≥2%) which occurred at a higher rate than the comparator are insomnia, headache, nausea, asthenia and fatigue (6.1).
- Creatine kinase elevations were observed in subjects who received ISENTRESS. Myopathy and rhabdomyolysis have been reported; however, the relationship of ISENTRESS to these events is not known. Use with caution in patients at increased risk of myopathy or rhabdomyolysis, such as patients receiving concomitant medications known to cause these conditions (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Merck & Co., Inc. at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Coadministration of ISENTRESS with drugs that are strong inducers of UGT1A1 may result in reduced plasma concentrations of raltegravir (7.2).
USE IN SPECIFIC POPULATIONS
Pregnancy:
- ISENTRESS should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Physicians are encouraged to register pregnant women exposed to ISENTRESS by calling 1-800-258-4263 so that Merck can monitor maternal and fetal outcomes (8.1).
Nursing Mothers:
- Breast-feeding is not recommended while taking ISENTRESS (8.3).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2010
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Immune Reconstitution Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Raltegravir on the Pharmacokinetics of Other Agents
7.2 Effect of Other Agents on the Pharmacokinetics of Raltegravir
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Patients with Hepatic Impairment
8.7 Use in Patients with Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
ISENTRESS1 is indicated in combination with other anti-retroviral agents for the treatment of human immunodeficiency virus (HIV-1) infection in adult patients.
This indication is based on analyses of plasma HIV-1 RNA levels up through 48 weeks in three double-blind controlled studies of ISENTRESS. Two of these studies were conducted in clinically advanced, 3-class antiretroviral (NNRTI, NRTI, PI) treatment-experienced adults and one was conducted in treatment-naïve adults.
The use of other active agents with ISENTRESS is associated with a greater likelihood of treatment response [see Clinical Studies (14)].
The safety and efficacy of ISENTRESS have not been established in pediatric patients.
- 1
Registered trademark of MERCK & CO., Inc.
COPYRIGHT © 2007, 2009 MERCK & CO., Inc.
All rights reserved - 1
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Immune Reconstitution Syndrome
During the initial phase of treatment, patients responding to antiretroviral therapy may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium complex, cytomegalovirus, Pneumocystis jiroveci pneumonia, Mycobacterium tuberculosis, or reactivation of varicella zoster virus), which may necessitate further evaluation and treatment.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Treatment-Naïve Studies
The following safety assessment of ISENTRESS in treatment-naïve subjects is based on the randomized double-blind active controlled study of treatment-naïve subjects, STARTMRK (Protocol 021) with ISENTRESS 400 mg twice daily in combination with a fixed dose of emtricitabine 200 mg (+) tenofovir 300 mg, (N=281) versus efavirenz (EFV) 600 mg at bedtime in combination with emtricitabine (+) tenofovir, (N=282). During double-blind treatment, the total follow-up for subjects receiving ISENTRESS 400 mg twice daily + emtricitabine (+) tenofovir was 247 patient-years and 241 patient-years for subjects receiving efavirenz 600 mg at bedtime + emtricitabine (+) tenofovir.
In Protocol 021, the rate of discontinuation of therapy due to adverse reactions was 3% in subjects receiving ISENTRESS + emtricitabine (+) tenofovir and 6% in subjects receiving efavirenz + emtricitabine (+) tenofovir.
The clinical adverse drug reactions (ADRs) listed below were considered by investigators to be causally related to ISENTRESS + emtricitabine (+) tenofovir or efavirenz + emtricitabine (+) tenofovir. Clinical ADRs of moderate to severe intensity occurring in ≥2% of treatment-naïve subjects treated with ISENTRESS and occurring at a higher rate than efavirenz are presented in Table 1.
Table 1: Adverse Reactions* of Moderate to Severe Intensity† Occurring in ≥2% of Treatment-Naïve Adult Subjects Receiving ISENTRESS and at a Higher Rate Compared to Efavirenz (48 Week Analysis) System Organ Class,
Preferred TermRandomized Study Protocol 021 ISENTRESS 400 mg
Twice Daily +
Emtricitabine (+) Tenofovir
(n = 281)‡
%Efavirenz 600 mg
At Bedtime +
Emtricitabine (+) Tenofovir
(n = 282)‡
%- * Includes adverse experiences considered by investigators to be at least possibly, probably, or definitely related to the drug
- † Intensities are defined as follows: Moderate (discomfort enough to cause interference with usual activity); Severe (incapacitating with inability to work or do usual activity).
- ‡ n = total number of subjects per treatment group
Psychiatric Disorders Insomnia 4 3 Less Common Adverse Reactions
The following ADRs occurred in <2% of subjects receiving ISENTRESS + emtricitabine (+) tenofovir. These events have been included because of their seriousness, increased frequency on ISENTRESS compared with efavirenz or investigator's assessment of potential causal relationship.
General Disorders and Administration Site Conditions: fatigue
Psychiatric Disorders: abnormal dreams
Laboratory Abnormalities
The percentages of adult subjects treated with ISENTRESS 400 mg twice daily or efavirenz in Protocol 021 with selected Grades 2 to 4 laboratory abnormalities that represent a worsening from baseline are presented in Table 2.
Table 2: Selected Grade 2 to 4 Laboratory Abnormalities Reported in Treatment-Naïve Subjects (48 Week Analysis) Randomized Study Protocol 021 Laboratory Parameter Preferred Term (Unit) Limit ISENTRESS
400 mg
Twice Daily +
Emtricitabine
(+) Tenofovir
(N = 281)Efavirenz
600 mg
At Bedtime +
Emtricitabine
(+) Tenofovir
(N = 282)ULN = Upper limit of normal range Hematology Absolute neutrophil count (103/μL) Grade 2 0.75 - 0.999 3% 3% Grade 3 0.50 - 0.749 1% <1% Grade 4 <0.50 <1% 0% Hemoglobin (gm/dL) Grade 2 7.5 - 8.4 <1% <1% Grade 3 6.5 - 7.4 <1% <1% Grade 4 <6.5 0% 0% Platelet count (103/μL) Grade 2 50 - 99.999 2% 0% Grade 3 25 - 49.999 0% <1% Grade 4 <25 0% .0% Blood chemistry Fasting (non-random) serum glucose test (mg/dL) Grade 2 126 - 250 2% 3% Grade 3 251 - 500 <1% 0% Grade 4 >500 0% 0% Total serum bilirubin Grade 2 1.6 - 2.5 x ULN 4% 0% Grade 3 2.6 - 5.0 x ULN <1% 0% Grade 4 >5.0 x ULN 0% 0% Serum aspartate aminotransferase Grade 2 2.6 - 5.0 x ULN 3% 4% Grade 3 5.1 - 10.0 x ULN 1% 1% Grade 4 >10.0 x ULN <1% <1% Serum alanine aminotransferase Grade 2 2.6 - 5.0 x ULN 4% 6% Grade 3 5.1 - 10.0 x ULN <1% 2% Grade 4 >10.0 x ULN <1% <1% Serum alkaline phosphatase Grade 2 2.6 - 5.0 x ULN <1% 2% Grade 3 5.1 - 10.0 x ULN 0% <1% Grade 4 >10.0 x ULN 0% 0% Lipids, Change from Baseline
Changes from baseline in fasting lipids are shown in Table 3.
Table 3: Lipid Values, Mean Change from Baseline, Protocol 021 Laboratory Parameter Preferred Term
ISENTRESS 400 mg
Twice Daily + Emtricitabine (+) Tenofovir
N = 281Efavirenz 600 mg
At Bedtime + Emtricitabine (+) Tenofovir
N = 282Change from
Baseline at
Week 48Change from
Baseline at
Week 48Baseline Mean(mg/dL) Week 48 Mean(mg/dL) Mean Change
(mg/dL)Baseline Mean(mg/dL) Week 48 Mean(mg/dL) Mean Change
(mg/dL)Notes: N = Number of subjects in the treatment group. The analysis is based on all available data. If subjects initiated or increased serum lipid-reducing agents, the last available lipid values prior to the change in therapy were used in the analysis. If the missing data was due to other reasons, subjects were censored thereafter for the analysis.
At baseline, serum lipid-reducing agents were used in 5% of subjects in the group receiving ISENTRESS and 3% in the efavirenz group. Through Week 48, serum lipid-reducing agents were used in 6% of subjects in the group receiving ISENTRESS and 6% in the efavirenz group.- * Fasting (non-random) laboratory tests.
LDL-Cholesterol* 97 103 6 92 108 16 HDL-Cholesterol* 38 42 4 38 48 10 Total Cholesterol*
159 169 10 156 188 33 Triglyceride* 125 122 -3 136 174 37 Treatment-Experienced Studies
The safety assessment of ISENTRESS in treatment-experienced subjects is based on the pooled safety data from the randomized, double-blind, placebo-controlled trials, BENCHMRK 1 and BENCHMRK 2 (Protocols 018 and 019) in antiretroviral treatment-experienced HIV-1 infected adult subjects. A total of 462 subjects received the recommended dose of ISENTRESS 400 mg twice daily in combination with optimized background therapy (OBT) compared to 237 subjects taking placebo in combination with OBT. The median duration of therapy in these trials was 48 weeks for subjects receiving ISENTRESS and 38 weeks for subjects receiving placebo. The total exposure to ISENTRESS was 387 patient-years versus 156 patient-years on placebo. The rates of discontinuation due to adverse events were 2% in subjects receiving ISENTRESS and 3% in subjects receiving placebo.
Clinical ADRs were considered by investigators to be causally related to ISENTRESS + OBT or placebo + OBT. Clinical ADRs of moderate to severe intensity occurring in ≥2% of subjects treated with ISENTRESS and occurring at a higher exposure adjusted rate compared to placebo are presented in Table 4.
Table 4: Adverse Drug Reactions* of Moderate to Severe Intensity† Occurring in ≥2% of Treatment-Experienced Adult Subjects Receiving ISENTRESS and at a Higher Exposure Adjusted Rate Compared to Placebo (48 Week Analysis, Exposure Adjusted Incidence Rates) System Organ Class,
Adverse ReactionsRandomized Studies Protocol 018 and 019 ISENTRESS 400 mg Twice Daily
+ OBT
(n = 462)‡Placebo + OBT
(n = 237)‡Rate per 100 Patient-Years
Rate per 100 Patient-Years
- * Includes adverse reactions at least possibly, probably, or definitely related to the drug.
- † Intensities are defined as follows: Moderate (discomfort enough to cause interference with usual activity); Severe (incapacitating with inability to work or do usual activity).
- ‡ n=total number of subjects per treatment group.
Nervous System Disorders Headache 3 1 Gastrointestinal Disorders Nausea 2 1 General Disorders and Administration Site Conditions Asthenia 2 1 Fatigue 2 1 Less Common Adverse Reactions
The following ADRs occurred in <2% of subjects receiving ISENTRESS + OBT. These events have been included because of either their seriousness, increased frequency on ISENTRESS compared with placebo or investigator's assessment of potential causal relationship.
Gastrointestinal Disorders: abdominal pain, gastritis
Hepatobiliary Disorders: hepatitis
Immune System Disorders: hypersensitivity
Infections and Infestations: genital herpes, herpes zoster
Nervous System Disorders: dizziness
Renal and Urinary Disorders: renal failure
Laboratory Abnormalities
The percentages of adult subjects treated with ISENTRESS 400 mg twice daily or placebo in Protocols 018 and 019 with selected Grade 2 to 4 laboratory abnormalities representing a worsening from baseline are presented in Table 5.
Table 5: Selected Grade 2 to 4 Laboratory Abnormalities Reported in Treatment-Experienced Subjects (48 Week Analysis) Randomized Studies Protocol 018
and 019Laboratory
Parameter
Preferred Term
(Unit)Limit ISENTRESS
400 mg Twice Daily +
OBT
(N = 462)Placebo
+
OBT
(N = 237)ULN = Upper limit of normal range Hematology Absolute neutrophil count (103/μL) Grade 2 0.75 - 0.999 3% 5% Grade 3 0.50 - 0.749 3% 3% Grade 4 <0.50 1% <1% Hemoglobin (gm/dL) Grade 2 7.5 - 8.4 1% 3% Grade 3 6.5 - 7.4 1% <1% Grade 4 <6.5 <1% 0% Platelet count (103/μL) Grade 2 50 - 99.999 3% 5% Grade 3 25 - 49.999 1% <1% Grade 4 <25 1% <1% Blood chemistry Fasting (non-random) serum glucose test (mg/dL) Grade 2 126 – 250 8% 5% Grade 3 251 – 500 2% 1% Grade 4 >500 0% 0% Total serum bilirubin Grade 2 1.6 - 2.5 x ULN 5% 3% Grade 3 2.6 - 5.0 x ULN 2% 2% Grade 4 >5.0 x ULN 1% 0% Serum aspartate aminotransferase Grade 2 2.6 - 5.0 x ULN 8% 6% Grade 3 5.1 - 10.0 x ULN 3% 3% Grade 4 >10.0 x ULN <1% 1% Serum alanine aminotransferase Grade 2 2.6 - 5.0 x ULN 7% 8% Grade 3 5.1 - 10.0 x ULN 3% 2% Grade 4 >10.0 x ULN 1% 2% Serum alkaline phosphatase Grade 2 2.6 - 5.0 x ULN 2% <1% Grade 3 5.1 - 10.0 x ULN <1% 1% Grade 4 >10.0 x ULN 1% <1% Serum pancreatic amylase test Grade 2 1.6 - 2.0 x ULN 2% 1% Grade 3 2.1 - 5.0 x ULN 3% 3% Grade 4 >5.0 x ULN <1% 0% Serum lipase test Grade 2 1.6 - 3.0 x ULN 4% 3% Grade 3 3.1 - 5.0 x ULN 1% <1% Grade 4 >5.0 x ULN 0% 0% Serum creatine kinase Grade 2 6.0 - 9.9 x ULN 2% 2% Grade 3 10.0 - 19.9 x ULN 3% 3% Grade 4 ≥20.0 x ULN 2% 1% Selected Adverse Events
Regardless of Drug Relationship
Cancers were reported in treatment-experienced subjects who initiated ISENTRESS or placebo, both with OBT, and in treatment-naïve subjects who initiated ISENTRESS or efavirenz, both with emtricitabine (+) tenofovir; several were recurrent. The types and rates of specific cancers were those expected in a highly immunodeficient population (many had CD4+ counts below 50 cells/mm3 and most had prior AIDS diagnoses). The risk of developing cancer in these studies was similar in the group receiving ISENTRESS and the group receiving the comparator.
Grade 2-4 creatine kinase laboratory abnormalities were observed in subjects treated with ISENTRESS (see Table 5). Myopathy and rhabdomyolysis have been reported; however, the relationship of ISENTRESS to these events is not known. Use with caution in patients at increased risk of myopathy or rhabdomyolysis, such as patients receiving concomitant medications known to cause these conditions.
Patients with Co-existing Conditions
Patients Co-infected with Hepatitis B and/or Hepatitis C Virus
In the randomized, double-blind, placebo-controlled trials, treatment-experienced subjects (N = 114/699 or 16%) and treatment-naïve subjects (N = 34/563 or 6%) with chronic (but not acute) active hepatitis B and/or hepatitis C virus co-infection were permitted to enroll provided that baseline liver function tests did not exceed 5 times the upper limit of normal (ULN). In general the safety profile of ISENTRESS in subjects with hepatitis B and/or hepatitis C virus co-infection was similar to that in subjects without hepatitis B and/or hepatitis C virus co-infection, although the rates of AST and ALT abnormalities were higher in the subgroup with hepatitis B and/or hepatitis C virus co-infection for all treatment groups. In treatment-experienced subjects, Grade 2 or higher laboratory abnormalities that represent a worsening Grade from baseline of AST, ALT or total bilirubin occurred in 25%, 31% and 12%, respectively, of co-infected subjects treated with ISENTRESS as compared to 8%, 7% and 8% of all other subjects treated with ISENTRESS. In treatment-naïve subjects, Grade 2 or higher laboratory abnormalities that represent a worsening Grade from baseline of AST, ALT or total bilirubin occurred in 17%, 22% and 11%, respectively, of co-infected subjects treated with ISENTRESS as compared to 4%, 4% and 3% of all other subjects treated with ISENTRESS.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ISENTRESS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Psychiatric Disorders: anxiety, depression (particularly in patients with a pre-existing history of psychiatric illness), including suicidal ideation and behaviors, paranoia
Skin and Subcutaneous Tissue Disorders: rash, Stevens-Johnson syndrome
-
7 DRUG INTERACTIONS
7.1 Effect of Raltegravir on the Pharmacokinetics of Other Agents
Raltegravir does not inhibit (IC50>100 µM) CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP3A in vitro. Moreover, in vitro, raltegravir did not induce CYP1A2, CYP2B6 or CYP3A4. A midazolam drug interaction study confirmed the low propensity of raltegravir to alter the pharmacokinetics of agents metabolized by CYP3A4 in vivo by demonstrating a lack of effect of raltegravir on the pharmacokinetics of midazolam, a sensitive CYP3A4 substrate. Similarly, raltegravir is not an inhibitor (IC50>50 µM) of the UDP-glucuronosyltransferases (UGT) tested (UGT1A1, UGT2B7), and raltegravir does not inhibit P-glycoprotein-mediated transport. Based on these data, ISENTRESS is not expected to affect the pharmacokinetics of drugs that are substrates of these enzymes or P-glycoprotein (e.g., protease inhibitors, NNRTIs, opioid analgesics, statins, azole antifungals, proton pump inhibitors and anti-erectile dysfunction agents).
In drug interaction studies, raltegravir did not have a clinically meaningful effect on the pharmacokinetics of the following: hormonal contraceptives, methadone, lamivudine, tenofovir, etravirine.
7.2 Effect of Other Agents on the Pharmacokinetics of Raltegravir
Raltegravir is not a substrate of cytochrome P450 (CYP) enzymes. Based on in vivo and in vitro studies, raltegravir is eliminated mainly by metabolism via a UGT1A1-mediated glucuronidation pathway.
Rifampin, a strong inducer of UGT1A1, reduces plasma concentrations of ISENTRESS. Therefore, the dose of ISENTRESS should be increased during coadministration with rifampin [see Dosage and Administration (2)]. The impact of other inducers of drug metabolizing enzymes, such as phenytoin and phenobarbital, on UGT1A1 is unknown.
Coadministration of ISENTRESS with drugs that inhibit UGT1A1 may increase plasma levels of raltegravir.
Selected drug interactions are presented in Table 6 [see Clinical Pharmacology (12.3)].
Table 6: Selected Drug Interactions Concomitant Drug Class:
Drug NameEffect on
Concentration
of RaltegravirClinical Comment HIV-1-Antiviral Agents atazanavir ↑ Atazanavir, a strong inhibitor of UGT1A1, increases plasma concentrations of raltegravir. However, since concomitant use of ISENTRESS with atazanavir/ritonavir did not result in a unique safety signal in Phase 3 studies, no dose adjustment is recommended. atazanavir/ritonavir ↑ Atazanavir/ritonavir increases plasma concentrations of raltegravir. However, since concomitant use of ISENTRESS with atazanavir/ritonavir did not result in a unique safety signal in Phase 3 studies, no dose adjustment is recommended. efavirenz ↓ Efavirenz reduces plasma concentrations of raltegravir. The clinical significance of this interaction has not been directly assessed. etravirine ↓ Etravirine reduces plasma concentrations of raltegravir. The clinical significance of this interaction has not been directly assessed. tipranavir/ritonavir ↓ Tipranavir/ritonavir reduces plasma concentrations of raltegravir. However, since comparable efficacy was observed for this combination relative to other ISENTRESS-containing regimens in Phase 3 studies 018 and 019, no dose adjustment is recommended. Other Agents omeprazole ↑ Coadministration of medicinal products that increase gastric pH (e.g., omeprazole) may increase raltegravir levels based on increased raltegravir solubility at higher pH. However, since concomitant use of ISENTRESS with proton pump inhibitors and H2 blockers did not result in a unique safety signal in Phase 3 studies, no dose adjustment is recommended. rifampin ↓ Rifampin, a strong inducer of UGT1A1, reduces plasma concentrations of raltegravir. The recommended dosage of ISENTRESS is 800 mg twice daily during coadministration with rifampin. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
ISENTRESS should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. There are no adequate and well-controlled studies in pregnant women. In addition, there have been no pharmacokinetic studies conducted in pregnant patients.
Developmental toxicity studies were performed in rabbits (at oral doses up to 1000 mg/kg/day) and rats (at oral doses up to 600 mg/kg/day). The reproductive toxicity study in rats was performed with pre-, peri-, and postnatal evaluation. The highest doses in these studies produced systemic exposures in these species approximately 3- to 4-fold the exposure at the recommended human dose. In both rabbits and rats, no treatment-related effects on embryonic/fetal survival or fetal weights were observed. In addition, no treatment-related external, visceral, or skeletal changes were observed in rabbits. However, treatment-related increases over controls in the incidence of supernumerary ribs were seen in rats at 600 mg/kg/day (exposures 3-fold the exposure at the recommended human dose).
Placenta transfer of drug was demonstrated in both rats and rabbits. At a maternal dose of 600 mg/kg/day in rats, mean drug concentrations in fetal plasma were approximately 1.5- to 2.5-fold greater than in maternal plasma at 1 hour and 24 hours postdose, respectively. Mean drug concentrations in fetal plasma were approximately 2% of the mean maternal concentration at both 1 and 24 hours postdose at a maternal dose of 1000 mg/kg/day in rabbits.
Antiretroviral Pregnancy Registry
To monitor maternal-fetal outcomes of pregnant patients exposed to ISENTRESS, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
8.3 Nursing Mothers
Breast-feeding is not recommended while taking ISENTRESS. In addition, it is recommended that HIV-1-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV-1.
It is not known whether raltegravir is secreted in human milk. However, raltegravir is secreted in the milk of lactating rats. Mean drug concentrations in milk were approximately 3-fold greater than those in maternal plasma at a maternal dose of 600 mg/kg/day in rats. There were no effects in rat offspring attributable to exposure of ISENTRESS through the milk.
8.4 Pediatric Use
Safety and effectiveness of ISENTRESS in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of ISENTRESS did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Use in Patients with Hepatic Impairment
No clinically important pharmacokinetic differences between subjects with moderate hepatic impairment and healthy subjects were observed. No dosage adjustment is necessary for patients with mild to moderate hepatic impairment. The effect of severe hepatic impairment on the pharmacokinetics of raltegravir has not been studied [see Clinical Pharmacology (12.3)].
8.7 Use in Patients with Renal Impairment
No clinically important pharmacokinetic differences between subjects with severe renal impairment and healthy subjects were observed. No dosage adjustment is necessary [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
No specific information is available on the treatment of overdosage with ISENTRESS. Doses as high as 1600-mg single dose and 800-mg twice-daily multiple doses were studied in healthy volunteers without evidence of toxicity. Occasional doses of 1800 mg per day were taken in the clinical studies of HIV-1 infected subjects without evidence of toxicity.
In the event of an overdose, it is reasonable to employ the standard supportive measures, e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring (including obtaining an electrocardiogram), and institute supportive therapy if required. The extent to which ISENTRESS may be dialyzable is unknown.
-
11 DESCRIPTION
ISENTRESS contains raltegravir potassium, a human immunodeficiency virus integrase strand transfer inhibitor. The chemical name for raltegravir potassium is N-[(4-Fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-4-pyrimidinecarboxamide monopotassium salt.
The empirical formula is C20H20FKN6O5 and the molecular weight is 482.51. The structural formula is:
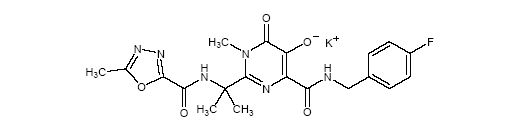
Raltegravir potassium is a white to off-white powder. It is soluble in water, slightly soluble in methanol, very slightly soluble in ethanol and acetonitrile and insoluble in isopropanol.
Each film-coated tablet of ISENTRESS for oral administration contains 434.4 mg of raltegravir potassium (as salt), equivalent to 400 mg of raltegravir (free phenol) and the following inactive ingredients: microcrystalline cellulose, lactose monohydrate, calcium phosphate dibasic anhydrous, hypromellose 2208, poloxamer 407 (contains 0.01% butylated hydroxytoluene as antioxidant), sodium stearyl fumarate, magnesium stearate. In addition, the film coating contains the following inactive ingredients: polyvinyl alcohol, titanium dioxide, polyethylene glycol 3350, talc, red iron oxide and black iron oxide.
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
In a monotherapy study raltegravir (400 mg twice daily) demonstrated rapid antiviral activity with mean viral load reduction of 1.66 log10 copies/mL by Day 10.
In the randomized, double-blind, placebo-controlled, dose-ranged trial, Protocol 005, and Protocols 018 and 019, antiviral responses were similar among subjects regardless of dose.
Effects on Electrocardiogram
In a randomized, placebo-controlled, crossover study, 31 healthy subjects were administered a single oral supratherapeutic dose of raltegravir 1600 mg and placebo. Peak raltegravir plasma concentrations were approximately 4-fold higher than the peak concentrations following a 400 mg dose. ISENTRESS did not appear to prolong the QTc interval for 12 hours postdose. After baseline and placebo adjustment, the maximum mean QTc change was -0.4 msec (1-sided 95% upper Cl: 3.1 msec).
12.3 Pharmacokinetics
Absorption
Raltegravir is absorbed with a Tmax of approximately 3 hours postdose in the fasted state. Raltegravir AUC and Cmax increase dose proportionally over the dose range 100 mg to 1600 mg. Raltegravir C12hr increases dose proportionally over the dose range of 100 to 800 mg and increases slightly less than dose proportionally over the dose range 100 mg to 1600 mg. With twice-daily dosing, pharmacokinetic steady state is achieved within approximately the first 2 days of dosing. There is little to no accumulation in AUC and Cmax. The average accumulation ratio for C12hr ranged from approximately 1.2 to 1.6.
The absolute bioavailability of raltegravir has not been established.
In subjects who received 400 mg twice daily alone, raltegravir drug exposures were characterized by a geometric mean AUC0-12hr of 14.3 μM●hr and C12hr of 142 nM.
Considerable variability was observed in the pharmacokinetics of raltegravir. For observed C12hr in Protocols 018 and 019, the coefficient of variation (CV) for inter-subject variability = 212% and the CV for intra-subject variability = 122%.
Effect of Food on Oral Absorption
ISENTRESS may be administered with or without food. Raltegravir was administered without regard to food in the pivotal safety and efficacy studies in HIV-1-infected patients. The effect of consumption of low-, moderate- and high-fat meals on steady-state raltegravir pharmacokinetics was assessed in healthy volunteers. Administration of multiple doses of raltegravir following a moderate-fat meal (600 Kcal, 21 g fat) did not affect raltegravir AUC to a clinically meaningful degree with an increase of 13% relative to fasting. Raltegravir C12hr was 66% higher and Cmax was 5% higher following a moderate-fat meal compared to fasting. Administration of raltegravir following a high-fat meal (825 Kcal, 52 g fat) increased AUC and Cmax by approximately 2-fold and increased C12hr by 4.1-fold. Administration of raltegravir following a low-fat meal (300 Kcal, 2.5 g fat) decreased AUC and Cmax by 46% and 52%, respectively; C12hr was essentially unchanged. Food appears to increase pharmacokinetic variability relative to fasting.
Distribution
Raltegravir is approximately 83% bound to human plasma protein over the concentration range of 2 to 10 µM.
Metabolism and Excretion
The apparent terminal half-life of raltegravir is approximately 9 hours, with a shorter α-phase half-life (~1 hour) accounting for much of the AUC. Following administration of an oral dose of radiolabeled raltegravir, approximately 51 and 32% of the dose was excreted in feces and urine, respectively. In feces, only raltegravir was present, most of which is likely derived from hydrolysis of raltegravir-glucuronide secreted in bile as observed in preclinical species. Two components, namely raltegravir and raltegravir-glucuronide, were detected in urine and accounted for approximately 9 and 23% of the dose, respectively. The major circulating entity was raltegravir and represented approximately 70% of the total radioactivity; the remaining radioactivity in plasma was accounted for by raltegravir-glucuronide. Studies using isoform-selective chemical inhibitors and cDNA-expressed UDP-glucuronosyltransferases (UGT) show that UGT1A1 is the main enzyme responsible for the formation of raltegravir-glucuronide. Thus, the data indicate that the major mechanism of clearance of raltegravir in humans is UGT1A1-mediated glucuronidation.
Special Populations
Pediatric
The pharmacokinetics of raltegravir in pediatric patients has not been established.
Age
The effect of age on the pharmacokinetics of raltegravir was evaluated in the composite analysis. No dosage adjustment is necessary.
Race
The effect of race on the pharmacokinetics of raltegravir was evaluated in the composite analysis. No dosage adjustment is necessary.
Gender
A study of the pharmacokinetics of raltegravir was performed in young healthy males and females. Additionally, the effect of gender was evaluated in a composite analysis of pharmacokinetic data from 103 healthy subjects and 28 HIV-1 infected subjects receiving raltegravir monotherapy with fasted administration. No dosage adjustment is necessary.
Hepatic Impairment
Raltegravir is eliminated primarily by glucuronidation in the liver. A study of the pharmacokinetics of raltegravir was performed in subjects with moderate hepatic impairment. Additionally, hepatic impairment was evaluated in the composite pharmacokinetic analysis. There were no clinically important pharmacokinetic differences between subjects with moderate hepatic impairment and healthy subjects. No dosage adjustment is necessary for patients with mild to moderate hepatic impairment. The effect of severe hepatic impairment on the pharmacokinetics of raltegravir has not been studied.
Renal Impairment
Renal clearance of unchanged drug is a minor pathway of elimination. A study of the pharmacokinetics of raltegravir was performed in subjects with severe renal impairment. Additionally, renal impairment was evaluated in the composite pharmacokinetic analysis. There were no clinically important pharmacokinetic differences between subjects with severe renal impairment and healthy subjects. No dosage adjustment is necessary. Because the extent to which ISENTRESS may be dialyzable is unknown, dosing before a dialysis session should be avoided.
UGT1A1 Polymorphism
There is no evidence that common UGT1A1 polymorphisms alter raltegravir pharmacokinetics to a clinically meaningful extent. In a comparison of 30 subjects with *28/*28 genotype (associated with reduced activity of UGT1A1) to 27 subjects with wild-type genotype, the geometric mean ratio (90% CI) of AUC was 1.41 (0.96, 2.09).
Drug Interactions [see Drug Interactions (7)]
Table 7: Effect of Other Agents on the Pharmacokinetics of Raltegravir Coadministered
DrugCoadministered
Drug Dose/ScheduleRaltegravir
Dose/ScheduleRatio (90% Confidence Interval) of
Raltegravir Pharmacokinetic Parameters
with/without Coadministered Drug;
No Effect = 1.00n Cmax AUC Cmin atazanavir 400 mg daily 100 mg single
dose10 1.53
(1.11, 2.12)1.72
(1.47, 2.02)1.95
(1.30, 2.92)atazanavir/ritonavir 300 mg/100 mg daily 400 mg twice
daily10 1.24
(0.87, 1.77)1.41
(1.12, 1.78)1.77
(1.39, 2.25)efavirenz 600 mg daily 400 mg single dose 9 0.64
(0.41, 0.98)0.64
(0.52, 0.80)0.79
(0.49, 1.28)etravirine 200 mg twice daily 400 mg twice daily 19 0.89
(0.68, 1.15)0.90
(0.68, 1.18)0.66
(0.34, 1.26)omeprazole 20 mg daily 400 mg single dose 14
(10 for AUC)4.15
(2.82, 6.10)3.12
(2.13, 4.56)1.46
(1.10, 1.93)rifampin 600 mg daily 400 mg single
dose9 0.62
(0.37, 1.04)0.60
(0.39, 0.91)0.39
(0.30, 0.51)rifampin 600 mg daily 400 mg twice daily when
administered alone;
800 mg twice daily when
administered with rifampin14 1.62
(1.12, 2.33)1.27
(0.94, 1.71)0.47
(0.36, 0.61)ritonavir 100 mg twice daily 400 mg single
dose10 0.76
(0.55, 1.04)0.84
(0.70, 1.01)0.99
(0.70 1.40)tenofovir 300 mg daily 400 mg twice daily 9 1.64
(1.16, 2.32)1.49
(1.15, 1.94)1.03
(0.73, 1.45)tipranavir/ritonavir 500 mg/200 mg twice daily 400 mg twice
daily15
(14 for Cmin)0.82
(0.46, 1.46)0.76
(0.49, 1.19)0.45
(0.31, 0.66)12.4 Microbiology
Mechanism of Action
Raltegravir inhibits the catalytic activity of HIV-1 integrase, an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the covalent insertion, or integration, of unintegrated linear HIV-1 DNA into the host cell genome preventing the formation of the HIV-1 provirus. The provirus is required to direct the production of progeny virus, so inhibiting integration prevents propagation of the viral infection. Raltegravir did not significantly inhibit human phosphoryltransferases including DNA polymerases α, β, and γ.
Antiviral Activity in Cell Culture
Raltegravir at concentrations of 31 ± 20 nM resulted in 95% inhibition (EC95) of viral spread (relative to an untreated virus-infected culture) in human T-lymphoid cell cultures infected with the cell-line adapted HIV-1 variant H9IIIB. In addition, 5 clinical isolates of HIV-1 subtype B had EC95 values ranging from 9 to 19 nM in cultures of mitogen-activated human peripheral blood mononuclear cells. In a single-cycle infection assay, raltegravir inhibited infection of 23 HIV-1 isolates representing 5 non-B subtypes (A, C, D, F, and G) and 5 circulating recombinant forms (AE, AG, BF, BG, and cpx) with EC50 values ranging from 5 to 12 nM. Raltegravir also inhibited replication of an HIV-2 isolate when tested in CEMx174 cells (EC95 value = 6 nM). Additive to synergistic antiretroviral activity was observed when human T-lymphoid cells infected with the H9IIIB variant of HIV-1 were incubated with raltegravir in combination with non-nucleoside reverse transcriptase inhibitors (delavirdine, efavirenz, or nevirapine); nucleoside analog reverse transcriptase inhibitors (abacavir, didanosine, lamivudine, stavudine, tenofovir, zalcitabine, or zidovudine); protease inhibitors (amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, or saquinavir); or the entry inhibitor enfuvirtide.
Resistance
The mutations observed in the HIV-1 integrase coding sequence that contributed to raltegravir resistance (evolved either in cell culture or in subjects treated with raltegravir) generally included an amino acid substitution at either Q148 (changed to H, K, or R) or N155 (changed to H) plus one or more additional substitutions (i.e., L74M, E92Q, T97A, E138A/K, G140A/S, V151I, G163R, H183P, Y226C/D/F/H, S230R and D232N). Amino acid substitution at Y143C/H/R is another pathway to raltegravir resistance.
Treatment-Naïve Subjects: By Week 48 in the STARTMRK trial, the primary raltegravir resistance-associated substitutions were observed in 3 (1 with Y143R and 2 with Q148H/R) of the 6 virologic failure subjects with evaluable paired genotypic data.
Treatment-Experienced Subjects: By Week 48 in the BENCHMRK trials, at least one of the 3 primary raltegravir resistance-associated substitutions, Y143C/H/R, Q148H/K/R, and N155H, was observed in 63 (64.3%) of the 98 virologic failure subjects with evaluable genotypic data from paired baseline and raltegravir treatment-failure isolates. Some (n=18) of those HIV-1 isolates harboring one or more of the 3 primary raltegravir resistance-associated substitutions were evaluated for raltegravir susceptibility yielding a median decrease of 47.3-fold (mean 73.1 ± 60.8-fold decrease, ranging from 0.9- to 200-fold) compared to baseline isolates.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies of raltegravir in mice did not show any carcinogenic potential. At the highest dose levels, 400 mg/kg/day in females and 250 mg/kg/day in males, systemic exposure was 1.8-fold (females) or 1.2-fold (males) greater than the AUC (54 µM●hr) at the 400-mg twice daily human dose. Treatment-related squamous cell carcinoma of nose/nasopharynx was observed in female rats dosed with 600 mg/kg/day raltegravir for 104 weeks. These tumors were possibly the result of local irritation and inflammation due to local deposition and/or aspiration of drug in the mucosa of the nose/nasopharynx during dosing. No tumors of the nose/nasopharynx were observed in rats dosed with 150 mg/kg/day (males) and 50 mg/kg/day (females) and the systemic exposure in rats was 1.7-fold (males) to 1.4-fold (females) greater than the AUC (54 μM●hr) at the 400-mg twice daily human dose.
No evidence of mutagenicity or genotoxicity was observed in in vitro microbial mutagenesis (Ames) tests, in vitro alkaline elution assays for DNA breakage and in vitro and in vivo chromosomal aberration studies.
No effect on fertility was seen in male and female rats at doses up to 600 mg/kg/day which resulted in 3.0-fold exposure above the exposure at the recommended human dose.
-
14 CLINICAL STUDIES
Description of Clinical Studies
The evidence of durable efficacy of ISENTRESS is based on the analyses of 48-week data from an ongoing, randomized, double-blind, active-control trial, STARTMRK (Protocol 021) in antiretroviral treatment-naive HIV-1 infected adult subjects and from 2 ongoing, randomized, double-blind, placebo-controlled studies, BENCHMRK 1 and BENCHMRK 2 (Protocols 018 and 019), in antiretroviral treatment-experienced HIV-1 infected adult subjects. These efficacy results were supported by the 96-week analysis of a randomized, double-blind, controlled, dose-ranging trial, Protocol 004, in antiretroviral treatment-naïve HIV-1 infected adult subjects and by the 48-week analysis of a randomized, double-blind, controlled, dose-ranging study, Protocol 005, in antiretroviral treatment-experienced HIV-1 infected adult subjects.
Treatment-Naïve Subjects
STARTMRK (Protocol 021) is a Phase 3 study to evaluate the safety and antiretroviral activity of ISENTRESS 400 mg twice daily + emtricitabine (+) tenofovir versus efavirenz 600 mg at bedtime plus emtricitabine (+) tenofovir in treatment-naïve HIV-1-infected subjects with HIV-1 RNA >5000 copies/mL. Randomization was stratified by screening HIV-1 RNA level (≤50,000 copies/mL; and >50,000 copies/mL) and by hepatitis status.
Table 8 shows the demographic characteristics of subjects in the group receiving ISENTRESS 400 mg twice daily and subjects in the comparator group.
Table 8: Baseline Characteristics Randomized Study ISENTRESS Efavirenz Protocol 021 400 mg Twice Daily 600 mg At Bedtime (N = 281) (N = 282) Notes:
ISENTRESS and Efavirenz were administered with emtricitabine (+) tenofovir
N = Number of subjects in each group.- * Includes additional subjects identified as having a history of AIDS.
- † Non-Clade B Subtypes (# of subjects): Clade A (4), A/C (1), A/G (2), A1 (1), AE (29), AG (12), BF (6), C (37), D (2), F (2), F1 (5), G (2), Complex (3).
- ‡ Evidence of hepatitis B surface antigen or evidence of HCV RNA by polymerase chain reaction (PCR) quantitative test for hepatitis C Virus.
Gender Male 81% 82% Female 19% 18% Race White 41% 44% Black 12% 8% Asian 13% 11% Hispanic 21% 24% Native American 0% 0% Multiracial 12% 13% Region Latin America 35% 34% Southeast Asia 12% 10% North America 29% 32% EU/Australia 23% 23% Age (years) 18-64 99% 99% ≥65 1% 1% Mean (SD) 38 (9) 37 (10) Median (min, max) 37 (19 to 67) 36 (19 to 71) CD4 Cell Count (cells/microL) Mean (SD) 219 (124) 217 (134) Median (min, max) 212 (1 to 620) 204 (4 to 807) Plasma HIV-1 RNA (log10 copies/mL) Mean (SD) 5 (1) 5 (1) Median (min, max) 5 (3 to 6) 5 (4 to 6) Plasma HIV-1 RNA (copies/mL) Geometric Mean 103205 106215 Median (min, max) 114000 (400 to 750000) 104000 (4410 to 750000) History of AIDS* Yes 18% 21% Viral Subtype Clade B 78% 82% Non-Clade B† 21% 17% Baseline Plasma HIV-1 RNA ≤100,000 copies/mL 45% 49% >100,000 copies/mL 55% 51% Baseline CD4 Cell Counts ≤50 cells/mm3 10% 11% >50 cells/mm3 and ≤ 200 cells/mm3 37% 37% >200 cells/mm3 53% 51% Hepatitis Status Hepatitis B or C Positive‡ 6% 6% Week 48 outcomes from Protocol 021 are shown in Table 9.
Table 9: Outcomes by Treatment Group through Week 48 Randomized Study
Protocol 021ISENTRESS
400 mgTwice Daily
(N = 281)Efavirenz
600 mg
At Bedtime
(N = 282)Difference
(ISENTRESS – Efavirenz)
(CI*)Outcome at Week 48 - * The 95% CI for treatment difference is adjusted by the screening HIV RNA level (<=50,000 copies/mL vs. >50,000 copies/mL) and Hepatitis B or C (negative vs. positive)
- † Other includes lack of efficacy, loss to follow-up, consent withdrawn, protocol violation and other
Subjects with HIV-1 RNA less than 50 copies/mL 87% 82% 4.7% (-1.3%, 10.6%) Subjects with HIV-1 RNA less than 400 copies/mL 91% 88% 3.6% (-1.5%, 8.7%) Mean CD4 cell count change from baseline (cells/mm3) 176 150 25.8 (5.0, 46.5) Virologic Failure (>50 copies/mL) 6% 7% Never suppressed through Week 48 and on study at Week 48 2% 3% Rebound 5% 5% Discontinued study drug 7% 10% Reasons for Discontinuation Death <1% 0% Adverse experiences 2% 5% Other† 4% 5% Treatment-Experienced Subjects
BENCHMRK 1 and BENCHMRK 2 are Phase 3 studies to evaluate the safety and antiretroviral activity of ISENTRESS 400 mg twice daily in combination with an optimized background therapy (OBT), versus OBT alone, in HIV-1-infected subjects, 16 years or older, with documented resistance to at least 1 drug in each of 3 classes (NNRTIs, NRTIs, PIs) of antiretroviral therapies. Randomization was stratified by degree of resistance to PI (1PI vs. >1PI) and the use of enfuvirtide in the OBT. Prior to randomization, OBT was selected by the investigator based on genotypic/phenotypic resistance testing and prior ART history.
Table 10 shows the demographic characteristics of subjects in the group receiving ISENTRESS 400 mg twice daily and subjects in the placebo group.
Table 10: Baseline Characteristics Randomized Studies
Protocol 018 and 019ISENTRESS 400 mg Twice Daily
+ OBT
(N = 462)Placebo
+ OBT
(N = 237)- * Hepatitis B virus surface antigen positive or hepatitis C virus antibody positive.
Gender Male 88% 89% Female 12% 11% Race White 65% 73% Black 14% 11% Asian 3% 3% Hispanic 11% 8% Others 6% 5% Age (years) Median (min, max) 45 (16 to 74) 45 (17 to 70) CD4+ Cell Count Median (min, max), cells/mm3 119 (1 to 792) 123 (0 to 759) ≤50 cells/mm3 32% 33% >50 and ≤200 cells/mm3 37% 36% Plasma HIV-1 RNA Median (min, max), log10 copies/mL 4.8 (2 to 6) 4.7 (2 to 6) >100,000 copies/mL 35% 33% History of AIDS Yes 92% 91% Prior Use of ART, Median (1st Quartile, 3rd Quartile) Years of ART Use 10 (7 to 12) 10 (8 to 12) Number of ART 12 (9 to 15) 12 (9 to 14) Hepatitis Co-infection* No Hepatitis B or C virus 83% 85% Hepatitis B virus only 8% 3% Hepatitis C virus only 8% 11% Co-infection of Hepatitis B and C virus 1% 1% Stratum Enfuvirtide in OBT 38% 38% Resistant to ≥2 PI 97% 95% Table 11 compares the characteristics of optimized background therapy at baseline in the group receiving ISENTRESS 400 mg twice daily and subjects in the control group.
Table 11: Characteristics of Optimized Background Therapy at Baseline Randomized Studies
Protocol 018 and 019ISENTRESS 400 mg Twice
Daily + OBT
(N = 462)Placebo + OBT
(N = 237)- * Darunavir use in OBT in darunavir naïve subjects was counted as one active PI.
- † The Phenotypic Sensitivity Score (PSS) and the Genotypic Sensitivity Score (GSS) were defined as the total oral ARTs in OBT to which a subject's viral isolate showed phenotypic sensitivity and genotypic sensitivity, respectively, based upon phenotypic and genotypic resistance tests. Enfuvirtide use in OBT in enfuvirtide-naïve subjects was counted as one active drug in OBT in the GSS and PSS. Similarly, darunavir use in OBT in darunavir-naïve subjects was counted as one active drug in OBT.
Number of ARTs in OBT Median (min, max) 4 (1 to 7) 4 (2 to 7) Number of Active PI in OBT by
Phenotypic Resistance Test*0 36% 41% 1 or more 60% 58% Phenotypic Sensitivity Score (PSS)† 0 15% 19% 1 31% 30% 2 31% 28% 3 or more 18% 20% Genotypic Sensitivity Score (GSS)† 0 25% 28% 1 39% 41% 2 24% 21% 3 or more 11% 10% Week 48 outcomes for the 699 subjects randomized and treated with the recommended dose of ISENTRESS 400 mg twice daily or placebo in the pooled BENCHMRK 1 and 2 studies are shown in Table 12.
Table 12: Outcomes by Treatment Group through Week 48
Randomized Studies
Protocol 018 and 019ISENTRESS 400 mg
Twice Daily
+ OBT
(N = 462)Placebo
+ OBT
(N = 237)Outcome at Week 48 - * The non-responders by Week 48 were defined by the protocol as those who did not achieve > 1.0 log10 HIV-1 RNA reduction and <400 HIV-1 RNA copies/mL starting at Week 16 or beyond.
- † Other includes lack of efficacy, loss to follow-up, consent withdrawn
Subjects with HIV-1 RNA less than 400 copies/mL 72% 37% Subjects with HIV-1 RNA less than 50 copies/mL 60% 31% Mean CD4 cell count change from baseline (cells/mm3) 106 44 Virologic Failure (>50 copies/mL) 36% 65% Never suppressed through Week 48 and on study at Week 48 11% 9% Rebound 13% 8% Non-responder by Week 48* 12% 48% Discontined study drug 4% 4% Reasons for Discontinuation Death 2% 2% Adverse Experiences <1% <1% Other† 2% 1% The mean changes in plasma HIV-1 RNA from baseline were -2.11 log10 copies/mL in the group receiving ISENTRESS 400 mg twice daily and -0.96 log10 copies/mL for the control group.
Treatment-emergent CDC Category C events occurred in 4% of the group receiving ISENTRESS 400 mg twice daily and 5% of the control group.
Virologic responses at Week 48 by baseline genotypic and phenotypic sensitivity score are shown in Table 13.
Table 13: Virologic Response at Week 48 by Baseline Genotypic/Phenotypic Sensitivity Score Randomized Studies
Protocol 018 and 019Percent with HIV-1 RNA
<400 copies/mL
at Week 48Percent with HIV-1 RNA
<50 copies/mL
at Week 48
nISENTRESS 400 mg
Twice Daily
+ OBT
(N = 462)
nPlacebo
+ OBT
(N = 237)
nISENTRESS 400 mg
Twice Daily
+ OBT
(N = 462)
nPlacebo
+ OBT
(N = 237)- * The Phenotypic Sensitivity Score (PSS) and the Genotypic Sensitivity Score (GSS) were defined as the total oral ARTs in OBT to which a subject's viral isolate showed phenotypic sensitivity and genotypic sensitivity, respectively, based upon phenotypic and genotypic resistance tests. Enfuvirtide use in OBT in enfuvirtide-naïve subjects was counted as one active drug in OBT in the GSS and PSS. Similarly, darunavir use in OBT in darunavir-naïve subjects was counted as one active drug in OBT.
Phenotypic Sensitivity Score (PSS)* 0 69 52 44 5 69 46 44 2 1 145 72 72 32 145 57 72 28 2 142 83 66 42 142 68 66 38 3 or more 85 72 48 60 85 67 48 46 Genotypic Sensitivity Score (GSS)* 0 115 50 66 8 115 43 66 3 1 178 79 96 38 178 63 96 35 2 111 85 49 65 111 70 49 53 3 or more 51 69 23 52 51 67 23 39 -
16 HOW SUPPLIED/STORAGE AND HANDLING
ISENTRESS tablets 400 mg are pink, oval-shaped, film-coated tablets with “227” on one side.
They are supplied by State of Florida DOH Central Pharmacy as follows:
NDC Strength Quantity/Form Color Source Prod. Code 53808-0650-1 400 mg 30 Tablets in a Blister Pack pink 0006-0227 Storage and Handling
Store at 20-25°C (68-77°F); excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature.
-
17 PATIENT COUNSELING INFORMATION
[See FDA-Approved Patient Labeling.]
Patients should be informed that ISENTRESS is not a cure for HIV infection or AIDS. They should also be told that people taking ISENTRESS may still get infections or other conditions common in people with HIV (opportunistic infections). Patients should also be told that it is very important that they stay under a physician's care during treatment with ISENTRESS.
Patients should be informed that ISENTRESS does not reduce the chance of passing HIV to others through sexual contact, sharing needles, or being exposed to blood. Patients should be advised to continue to practice safer sex and to use latex or polyurethane condoms or other barrier methods to lower the chance of sexual contact with any body fluids such as semen, vaginal secretions or blood. Patients should also be advised to never re-use or share needles.
Physicians should instruct their patients that if they miss a dose, they should take it as soon as they remember. If they do not remember until it is time for the next dose, they should be instructed to skip the missed dose and go back to the regular schedule. Patients should not take two tablets of ISENTRESS at the same time.
Physicians should instruct their patients to read the Patient Package Insert before starting ISENTRESS therapy and to reread each time the prescription is renewed. Patients should be instructed to inform their physician or pharmacist if they develop any unusual symptom, or if any known symptom persists or worsens.
Manufactured and Distributed by:
MERCK & CO., INC., Whitehouse Station, NJ 08889, USAU.S. Patent Nos. US 7,169,780
This Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States
-
PATIENT PACKAGE INSERT
FDA-Approved Patient LabelingPatient Information
ISENTRESS® (eye sen tris)
(raltegravir)
TabletsRead the patient information that comes with ISENTRESS1 before you start taking it and each time you get a refill. There may be new information. This leaflet is a summary of the information for patients. Your doctor or pharmacist can give you additional information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is ISENTRESS?
- ISENTRESS is an anti-HIV (antiretroviral) medicine used for the treatment of HIV. The term HIV stands for Human Immunodeficiency Virus. It is the virus that causes AIDS (Acquired Immune Deficiency Syndrome). ISENTRESS is used along with other anti-HIV medicines. ISENTRESS will NOT cure HIV infection.
- People taking ISENTRESS may still develop infections, including opportunistic infections or other conditions that happen with HIV infection.
- Stay under the care of your doctor during treatment with ISENTRESS.
- The safety and effectiveness of ISENTRESS in children has not been studied.
ISENTRESS must be used with other anti-HIV medicines.
How does ISENTRESS work?
- ISENTRESS blocks an enzyme which the virus (HIV) needs in order to make more virus. The enzyme that ISENTRESS blocks is called HIV integrase.
- When used with other anti-HIV medicines, ISENTRESS may do two things:
- Reduce the amount of HIV in your blood. This is called your "viral load".
- Increase the number of white blood cells called CD4 (T) cells.
- ISENTRESS may not have these effects in all patients.
Does ISENTRESS lower the chance of passing HIV to other people?
No. ISENTRESS does not reduce the chance of passing HIV to others through sexual contact, sharing needles, or being exposed to your blood.
- Continue to practice safer sex.
- Use latex or polyurethane condoms or other barrier methods to lower the chance of sexual contact with any body fluids. This includes semen from a man, vaginal secretions from a woman, or blood.
- Never re-use or share needles.
Ask your doctor if you have any questions about safer sex or how to prevent passing HIV to other people.
What should I tell my doctor before and during treatment with ISENTRESS?Tell your doctor about all of your medical conditions. Include any of the following that applies to you:
- You have any allergies.
- You are pregnant or plan to become pregnant.
- ISENTRESS is not recommended for use during pregnancy. ISENTRESS has not been studied in pregnant women. If you take ISENTRESS while you are pregnant, talk to your doctor about how you can be included in the Antiretroviral Pregnancy Registry.
- You are breast-feeding or plan to breast-feed.
- It is recommended that HIV-infected women should not breast-feed their infants. This is because their babies could be infected with HIV through their breast milk.
- Talk with your doctor about the best way to feed your baby.
Tell your doctor about all the medicines you take. Include the following:
- prescription medicines, including rifampin (a medicine used to treat some infections such as tuberculosis)
- non-prescription medicines
- vitamins
- herbal supplements
Know the medicines you take.
- Keep a list of your medicines. Show the list to your doctor and pharmacist when you get a new medicine.
How should I take ISENTRESS?
Take ISENTRESS exactly as your doctor has prescribed. The recommended dose is as follows:
- Take only one 400-mg tablet at a time.
- Take it twice a day.
- Take it by mouth.
- Take it with or without food.
Do not change your dose or stop taking ISENTRESS or your other anti-HIV medicines without first talking with your doctor.
IMPORTANT: Take ISENTRESS exactly as your doctor prescribed and at the right times of day because if you don't:
- The amount of virus (HIV) in your blood may increase if the medicine is stopped for even a short period of time.
- The virus may develop resistance to ISENTRESS and become harder to treat.
- Your medicines may stop working to fight HIV.
- The activity of ISENTRESS may be reduced (due to resistance).
If you fail to take ISENTRESS the way you should, here's what to do:
- If you miss a dose, take it as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule. Do NOT take two tablets of ISENTRESS at the same time. In other words, do NOT take a double dose.
- If you take too much ISENTRESS, call your doctor or local Poison Control Center.
Be sure to keep a supply of your anti-HIV medicines.
- When your ISENTRESS supply starts to run low, get more from your doctor or pharmacy.
- Do not wait until your medicine runs out to get more.
What are the possible side effects of ISENTRESS?
When ISENTRESS has been given with other anti-HIV drugs, the most common side effects included:
- nausea
- headache
- tiredness
- weakness
- trouble sleeping
Other side effects include rash, severe skin reactions, feeling anxious, depression, suicidal thoughts and actions, paranoia.
A condition called Immune Reconstitution Syndrome can happen in some patients with advanced HIV infection (AIDS) when combination antiretroviral treatment is started. Signs and symptoms of inflammation from opportunistic infections that a person has or had may occur as the medicines work to treat the HIV infection and help to strengthen the immune system. Call your doctor right away if you notice any signs or symptoms of an infection after starting ISENTRESS with other anti-HIV medicines.
Contact your doctor promptly if you experience unexplained muscle pain, tenderness, or weakness while taking ISENTRESS. This is because on rare occasions, muscle problems can be serious and can lead to kidney damage.
Tell your doctor if you have any side effects that bother you.
These are not all the side effects of ISENTRESS. For more information, ask your doctor or pharmacist.
How should I store ISENTRESS?
- Store ISENTRESS at room temperature (68 to 77°F).
- Keep ISENTRESS and all medicines out of the reach of children.
General information about the use of ISENTRESS
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets.
- Do not use ISENTRESS for a condition for which it was not prescribed.
- Do not give ISENTRESS to other people, even if they have the same symptoms you have. It may harm them.
This leaflet gives you the most important information about ISENTRESS.
- If you would like to know more, talk with your doctor.
- You can ask your doctor or pharmacist for additional information about ISENTRESS that is written for health professionals.
- For more information go to www.ISENTRESS.com or call 1-800-622-4477.
What are the ingredients in ISENTRESS?
Active ingredient: Each film-coated tablet contains 400 mg of raltegravir.
Inactive ingredients: Microcrystalline cellulose, lactose monohydrate, calcium phosphate dibasic anhydrous, hypromellose 2208, poloxamer 407 (contains 0.01% butylated hydroxytoluene as antioxidant), sodium stearyl fumarate, magnesium stearate. In addition, the film coating contains the following inactive ingredients: polyvinyl alcohol, titanium dioxide, polyethylene glycol 3350, talc, red iron oxide and black iron oxide.
Manufactured and Distributed by:
MERCK & CO., Inc.
Whitehouse Station, NJ 08889, USAU.S. Patent Nos. US 7,169,780
This Product was Repackaged By:
State of Florida DOH Central Pharmacy
104-2 Hamilton Park Drive
Tallahassee, FL 32304
United States
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 400 mg
Isentress™
(raltegravir) tablets400 mg
Each tablet contains 434.4 mg raltegravir potassium, equivalent to 400 mg raltegravir.
Rx only
60 Tablets
NDC: 53808-0650-1
Store at 20-25°C (68-77°F); excursions permitted to 15-30°C (59-86°F). See USP Controlled Room Temperature.
USUAL ADULT DOSAGE:
See accompanying circular.MERCK & CO., INC.
Whitehouse Station, NJ 08889, USARaltegravir potassium (active ingred.)
Made in IrelandFormulated in USA

-
INGREDIENTS AND APPEARANCE
ISENTRESS
raltegravir tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 53808-0650(NDC:0006-0227) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RALTEGRAVIR POTASSIUM (UNII: 43Y000U234) (RALTEGRAVIR - UNII:22VKV8053U) RALTEGRAVIR 400 mg Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) HYPROMELLOSE (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLOXAMER 407 (UNII: TUF2IVW3M2) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYVINYL ALCOHOL (UNII: 532B59J990) FERRIC OXIDE RED (UNII: 1K09F3G675) SODIUM STEARYL FUMARATE (UNII: 7CV7WJK4UI) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK (pink) Score no score Shape OVAL (oval-shaped) Size 16mm Flavor Imprint Code 227 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53808-0650-1 30 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022145 07/01/2009 Labeler - State of Florida DOH Central Pharmacy (829348114) Establishment Name Address ID/FEI Business Operations State of Florida DOH Central Pharmacy 829348114 repack
Trademark Results [ISENTRESS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ISENTRESS 78866853 3357758 Live/Registered |
MERCK SHARP & DOHME CORP. 2006-04-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.