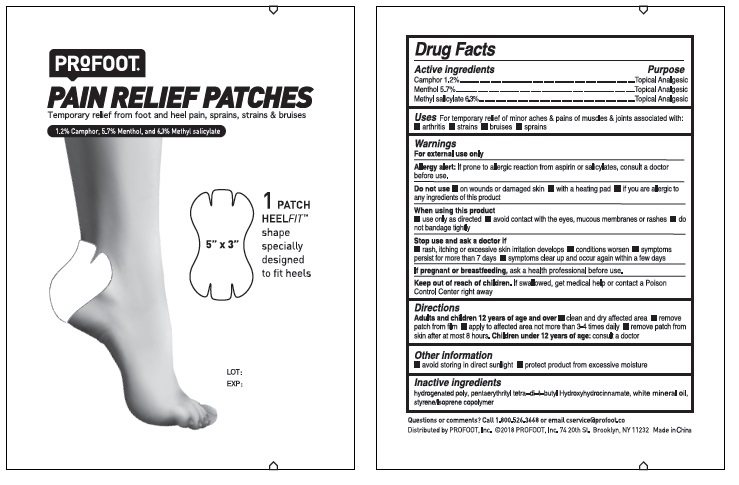
PROFOOT PAIN RELIEF PATCHES- camphor, menthol, methyl salicylate kit
Profoot Pain Relief Patches by
Drug Labeling and Warnings
Profoot Pain Relief Patches by is a Otc medication manufactured, distributed, or labeled by Profoot, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
Do not use
on wounds or damaged skin with a heating pad if you are allergic to any of the ingredients of
this product
When using this product
use only as directed avoid contact with the eyes, mucous membranes or rashes do not
bandage tightly
Stop use and ask a doctor if
rash, itching or excessive skin irritation develops condition worsens symptoms persist
for more than 7 days symptoms clear up and occur again within a few days
If pregnant or breastfeeding, ask a health professional before use.
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PROFOOT PAIN RELIEF PATCHES
camphor, menthol, methyl salicylate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 29784-600 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 29784-600-01 1 in 1 KIT 09/26/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PATCH 0.36 g Part 2 1 PATCH 1.3 g Part 1 of 2 PROFOOT
camphor, menthol, methyl salicylate patchProduct Information Item Code (Source) NDC: 29784-121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 12 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 57 mg in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 63 mg in 1 g Inactive Ingredients Ingredient Name Strength ALUMINUM SILICATE (UNII: T1FAD4SS2M) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PARAFFIN (UNII: I9O0E3H2ZE) ROSIN (UNII: 88S87KL877) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) STYRENE (UNII: 44LJ2U959V) ISOPRENE (UNII: 0A62964IBU) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4 in 1 KIT 1 NDC: 29784-121-36 0.36 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/26/2018 Part 2 of 2 PROFOOT
camphor, menthol, methyl salicylate patchProduct Information Item Code (Source) NDC: 29784-122 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 12 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 57 mg in 1 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 63 mg in 1 g Inactive Ingredients Ingredient Name Strength ALUMINUM SILICATE (UNII: T1FAD4SS2M) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PARAFFIN (UNII: I9O0E3H2ZE) ROSIN (UNII: 88S87KL877) POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) STYRENE (UNII: 44LJ2U959V) ISOPRENE (UNII: 0A62964IBU) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 2 in 1 KIT 1 NDC: 29784-122-36 1.3 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/26/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/26/2018 Labeler - Profoot, Inc. (107570900) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co., Ltd. 529128763 manufacture(29784-600)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.