FLUVASTATIN SODIUM tablet, film coated, extended release
Fluvastatin Sodium by
Drug Labeling and Warnings
Fluvastatin Sodium by is a Prescription medication manufactured, distributed, or labeled by Teva Pharmaceuticals USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FLUVASTATIN SODIUM EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for FLUVASTATIN SODIUM EXTENDED-RELEASE TABLETS.
FLUVASTATIN SODIUM extended-release tablets, for oral use
Initial U.S. Approval: 2000INDICATIONS AND USAGE
Fluvastatin sodium extended-release tablets are an HMG-CoA reductase inhibitor (statin) indicated as an adjunctive therapy to diet to:
- Reduce elevated TC, LDL-C, Apo B, and TG, and to increase HDL-C in adult patients with primary hypercholesterolemia and mixed dyslipidemia (1.1)
- Reduce elevated TC, LDL-C, and Apo B levels in boys and post-menarchal girls, 10 to 16 years of age, with heterozygous familial hypercholesterolemia after failing an adequate trial of diet therapy (1.1)
- Reduce the risk of undergoing revascularization procedures in patients with clinically evident CHD (1.2)
- Slow the progression of atherosclerosis in patients with CHD (1.2)
Limitations of Use:
- Fluvastatin sodium extended-release tablets have not been studied in conditions where the major abnormality is elevation of chylomicrons, VLDL, or IDL (i.e., hyperlipoproteinemia Types I, III, IV, or V). (1.3)
DOSAGE AND ADMINISTRATION
- Dose range: 20 mg to 80 mg/day. (2.1)
- Fluvastatin sodium extended-release tablets can be taken with or without food and may be taken at any time of the day. (2.1)
- Do not break, crush or chew fluvastatin sodium extended-release tablets prior to administration. (2.1)
- Adults: the recommended starting dose is 80 mg (administered as one 80 mg fluvastatin sodium extended-release tablet once daily). (2.2)
- Children with heterozygous familial hypercholesterolemia (ages 10 to 16, inclusive): the recommended starting dose is fluvastatin capsule 20 mg once daily. (2.3)
DOSAGE FORMS AND STRENGTHS
Fluvastatin sodium extended-release tablets: 80 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Skeletal muscle effects (e.g., myopathy and rhabdomyolysis): Risks increase with advanced age (≥ 65), uncontrolled hypothyroidism, renal impairment, and combination use with cyclosporine, or gemfibrozil. Advise patients to promptly report to their physician unexplained and/or persistent muscle pain, tenderness, or weakness and discontinue fluvastatin sodium extended-release tablets if myopathy is diagnosed or suspected. (5.1, 8.5, 8.7)
- Patients should be advised to report promptly any symptoms of myopathy. Fluvastatin sodium extended-release tablet therapy should be discontinued if myopathy is diagnosed or suspected (5.1)
- Liver enzyme abnormalities: Persistent elevations in hepatic transaminases can occur. Check liver enzyme tests before initiating therapy and as clinically indicated thereafter (5.2)
ADVERSE REACTIONS
Most frequent adverse reactions (rate ≥ 2% and > placebo) are: headache, dyspepsia, myalgia, abdominal pain and nausea (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact TEVA USA, PHARMACOVIGILANCE at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Cyclosporine: Combination increases fluvastatin exposure. Limit fluvastatin dose to 20 mg. (2.4, 7.1)
- Fluconazole: Combination increases fluvastatin exposure. Limit fluvastatin dose to 20 mg. (2.5, 7.2)
- Concomitant lipid-lowering therapies: Use with fibrates or lipid-modifying doses (≥ 1 g/day) of niacin increases the risk of adverse skeletal muscle effects. Caution should be used when prescribing with fluvastatin sodium extended-release tablets. (5.1, 7.3, 7.4)
- Glyburide: Monitor blood glucose levels when fluvastatin dose is changed. (7)
- Phenytoin: Monitor plasma phenytoin levels when fluvastatin treatment is initiated or when the dosage is changed. (7)
- Warfarin and coumarin derivates: Monitor prothrombin times when fluvastatin coadministration is initiated, discontinued, or the dosage changed. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
1.2 Secondary Prevention of Cardiovascular Disease
1.3 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Adult Patients With Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
2.3 Pediatric Patients (10 to 16 Years of Age) With Heterozygous Familial Hypercholesterolemia
2.4 Use With Cyclosporine
2.5 Use With Fluconazole
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity to any Component of This Medication
4.2 Active Liver Disease
4.3 Pregnancy
4.4 Nursing Mothers
5 WARNINGS AND PRECAUTIONS
5.1 Skeletal Muscle
5.2 Liver Enzymes
5.3 Endocrine Effects
5.4 CNS Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience in Adult Patients
6.2 Clinical Studies Experience in Pediatric Patients
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Cyclosporine
7.2 Fluconazole
7.3 Gemfibrozil
7.4 Other Fibrates
7.5 Niacin
7.6 Glyburide
7.7 Phenytoin
7.8 Warfarin
7.9 Colchicine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
14.2 Heterozygous Familial Hypercholesterolemia in Pediatric Patients
14.3 Secondary Prevention of Cardiovascular Disease
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
Store and Dispense
17 PATIENT COUNSELING INFORMATION
17.1 Muscle Pain
17.2 Liver Enzymes
17.3 Pregnancy
17.4 Breastfeeding
Fluvastatin Sodium Extended-Release Tablets 80 mg 30s Label Text
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Therapy with lipid-altering agents should be only one component of multiple risk factor intervention in individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Drug therapy is indicated as an adjunct to diet when the response to a diet restricted in saturated fat and cholesterol and other non-pharmacologic measures alone has been inadequate.
1.1 Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
Fluvastatin sodium extended-release tablets are indicated
- as an adjunct to diet to reduce elevated total cholesterol (Total-C), low-density lipoprotein cholesterol (LDL-C), triglyceride (TG) and apolipoprotein B (Apo B) levels, and to increase high-density lipoprotein cholesterol (HDL-C) in patients with primary hypercholesterolemia and mixed dyslipidemia (Fredrickson Type IIa and IIb).
- as an adjunct to diet to reduce Total-C, LDL-C, and Apo B levels in adolescent boys and adolescent girls who are at least one year post-menarche, 10 to 16 years of age, with heterozygous familial hypercholesterolemia and the following findings are present:
- LDL-C remains ≥ 190 mg/dL or
- LDL-C remains ≥ 160 mg/dL and:
- there is a positive family history of premature cardiovascular disease or
- two or more other cardiovascular disease risk factors are present
The NCEP classification of cholesterol levels in pediatric patients with a familial history of hypercholesterolemia or premature CVD is summarized below.
Category
Total-C (mg/dL)
LDL-C (mg/dL)
Acceptable
< 170
< 110
Borderline
170 to 199
110 to 129
High
≥ 200
≥ 130
Children treated with fluvastatin in adolescence should be re-evaluated in adulthood and appropriate changes made to their cholesterol-lowering regimen to achieve adult treatment goals.
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
Dose range: 20 mg to 80 mg/day.
Fluvastatin sodium extended-release tablets can be administered orally as a single dose, with or without food.
Do not break, crush or chew fluvastatin sodium extended-release tablets prior to administration.
Since the maximal effect of a given dose is seen within 4 weeks, periodic lipid determinations should be performed at this time and dosage adjusted according to the patient’s response to therapy and established treatment guidelines.
For patients requiring LDL-C reduction to a goal of ≥ 25%, the recommended starting dose is one fluvastatin sodium extended-release tablet, 80 mg administered as a single dose at any time of the day. For patients requiring LDL-C reduction to a goal of < 25% a starting dose of 20 mg may be used.
2.2 Adult Patients With Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
The recommended starting dose for fluvastatin sodium extended-release tablets is one 80 mg tablet administered as a single dose at any time of the day.
2.3 Pediatric Patients (10 to 16 Years of Age) With Heterozygous Familial Hypercholesterolemia
The recommended starting dose is one 20 mg fluvastatin capsule. Dose adjustments, up to a maximum daily dose administered as one fluvastatin sodium extended-release tablet, 80 mg once daily should be made at 6 week intervals. Doses should be individualized according to the goal of therapy [see NCEP Pediatric Panel Guidelines and CLINICAL STUDIES (14)]1.
1 National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 89(3):495-501. 1992.
2.4 Use With Cyclosporine
Do not exceed a dose of 20 mg b.i.d. fluvastatin capsules in patients taking cyclosporine [see Drug Interactions (7.1)].
2.5 Use With Fluconazole
Do not exceed a dose of 20 mg b.i.d. fluvastatin capsules in patients taking fluconazole [see Drug Interactions (7.2)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity to any Component of This Medication
Fluvastatin sodium extended-release tablets are contraindicated in patients with hypersensitivity to any component of this medication.
4.2 Active Liver Disease
Fluvastatin sodium extended-release tablets are contraindicated in patients with active liver disease or unexplained, persistent elevations in serum transaminases [see Warnings and Precautions (5.2)].
4.3 Pregnancy
Fluvastatin sodium extended-release tablets are contraindicated in women who are pregnant or may become pregnant. Serum cholesterol and triglycerides increase during normal pregnancy, and cholesterol or cholesterol derivatives are essential for fetal development. Fluvastatin sodium extended-release tablets may cause fetal harm when administered to pregnant women. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia.
Fluvastatin sodium extended-release tablets should be administered to women of childbearing age only when such patients are highly unlikely to conceive and have been informed of the potential hazards. If the patient becomes pregnant while taking this drug, fluvastatin sodium extended-release tablets should be discontinued and the patient should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
4.4 Nursing Mothers
Fluvastatin is secreted into the breast milk of animals and because HMG-CoA reductase inhibitors have the potential to cause serious adverse reactions in nursing infants, women who require treatment with fluvastatin sodium extended-release tablets should be advised not to breastfeed their infants [see Use in Specific Populations (8.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Skeletal Muscle
Rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with fluvastatin capsules/fluvastatin sodium extended-release tablets and other drugs in this class.
Fluvastatin sodium extended-release tablets should be prescribed with caution in patients with predisposing factors for myopathy. These factors include advanced age (> 65 years), renal impairment, and inadequately treated hypothyroidism.
The risk of myopathy and/or rhabdomyolysis with statins is increased with concurrent therapy with cyclosporine, erythromycin, fibrates or niacin. Myopathy was not observed in a clinical trial in 74 patients involving patients who were treated with fluvastatin sodium extended-release tablets together with niacin. Isolated cases of myopathy have been reported during postmarketing experience with concomitant administration of fluvastatin sodium extended-release tablets and colchicine. No information is available on the pharmacokinetic interaction between fluvastatin sodium extended-release tablets and colchicine.
Uncomplicated myalgia has also been reported in fluvastatin sodium treated patients [see Adverse Reactions (6)]. In clinical trials, uncomplicated myalgia has been observed infrequently in patients treated with fluvastatin sodium at rates indistinguishable from placebo. Myopathy, defined as muscle aching or muscle weakness in conjunction with increases in CPK values to greater than 10 times the upper limit of normal, was < 0.1% in fluvastatin clinical trials. Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevation of CPK.
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; improvement with immunosuppressive agents.
All patients should be advised to promptly report to their physician unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing fluvastatin sodium extended-release tablets.
Fluvastatin sodium extended-release tablet therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Fluvastatin sodium extended-release tablet therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
5.2 Liver Enzymes
Increases in serum transaminases (aspartate aminotransferase [AST]/serum glutamic-oxaloacetic transaminase, or alanine aminotransferase [ALT]/serum glutamic-pyruvic transaminase) have been reported with HMG-CoA reductase inhibitors, including fluvastatin sodium extended-release tablets. In most cases, the elevations were transient and resolved or improved on continued therapy or after a brief interruption in therapy.
Approximately 1.1% of patients treated with fluvastatin capsules in worldwide trials developed dose-related, persistent elevations of serum transaminase levels to more than 3 times the upper limit of normal. Fourteen of these patients (0.6%) were discontinued from therapy. In all clinical trials, a total of 33/2969 patients (1.1%) had persistent transaminase elevations with an average fluvastatin sodium exposure of approximately 71.2 weeks; 19 of these patients (0.6%) were discontinued. The majority of patients with these abnormal biochemical findings were asymptomatic.
In a pooled analysis of all placebo-controlled studies in which fluvastatin capsules were used, persistent transaminase elevations (> 3 times the upper limit of normal [ULN] on two consecutive weekly measurements) occurred in 0.2%, 1.5%, and 2.7% of patients treated with daily doses of 20, 40, and 80 mg (titrated to 40 mg twice daily) fluvastatin capsules, respectively. Ninety-one percent of the cases of persistent liver function test abnormalities (20 of 22 patients) occurred within 12 weeks of therapy and in all patients with persistent liver function test abnormalities there was an abnormal liver function test present at baseline or by Week 8.
In the pooled analysis of the 24 week controlled trials, persistent transaminase elevation occurred in 1.9%, 1.8% and 4.9% of patients treated with fluvastatin sodium extended-release tablets, 80 mg, fluvastatin capsules 40 mg and fluvastatin capsules 40 mg twice daily, respectively. In 13 of 16 patients treated with fluvastatin sodium extended-release tablets the abnormality occurred within 12 weeks of initiation of treatment with fluvastatin sodium extended-release tablets, 80 mg.
It is recommended that liver enzyme tests be performed prior to the initiation of fluvastatin sodium extended-release tablets, and if signs or symptoms of liver injury occur.
There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including fluvastatin. If serious liver injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs during treatment with fluvastatin sodium extended-release tablets, promptly interrupt therapy. If an alternate etiology is not found do not restart fluvastatin sodium extended-release tablets.
In very rare cases, possibly drug-related hepatitis was observed that resolved upon discontinuation of treatment.1 Active liver disease or unexplained serum transaminase elevations are contraindications to the use of fluvastatin sodium extended-release tablets [see Contraindications (4) and Warnings and Precautions (5.2)]. Caution should be exercised when fluvastatin sodium is administered to patients with a history of liver disease or heavy alcohol ingestion [see Clinical Pharmacology (12.3)]. Such patients should be closely monitored.
5.3 Endocrine Effects
Increases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including fluvastatin sodium extended-release tablets.
Statins interfere with cholesterol synthesis and lower circulating cholesterol levels and, as such, might theoretically blunt adrenal or gonadal steroid hormone production.
Fluvastatin sodium extended-release tablets exhibited no effect upon non-stimulated cortisol levels and demonstrated no effect upon thyroid metabolism as assessed by measurement of thyroid stimulating hormone (TSH). Small declines in total serum testosterone have been noted in treated groups, but no commensurate elevation in LH occurred, suggesting that the observation was not due to a direct effect upon testosterone production. No effect upon FSH in males was noted. Due to the limited number of premenopausal females studied to date, no conclusions regarding the effect of fluvastatin sodium extended-release tablets upon female sex hormones may be made.
Two clinical studies in patients receiving fluvastatin at doses up to 80 mg daily for periods of 24 to 28 weeks demonstrated no effect of treatment upon the adrenal response to ACTH stimulation. A clinical study evaluated the effect of fluvastatin at doses up to 80 mg daily for 28 weeks upon the gonadal response to HCG stimulation. Although the mean total testosterone response was significantly reduced (p < 0.05) relative to baseline in the 80 mg group, it was not significant in comparison to the changes noted in groups receiving either 40 mg of fluvastatin or placebo.
Patients treated with fluvastatin sodium extended-release tablets who develop clinical evidence of endocrine dysfunction should be evaluated appropriately. Caution should be exercised if a statin or other agent used to lower cholesterol levels is administered to patients receiving other drugs (e.g., ketoconazole, spironolactone, cimetidine) that may decrease the levels of endogenous steroid hormones.
5.4 CNS Toxicity
CNS effects, as evidenced by decreased activity, ataxia, loss of righting reflex, and ptosis were seen in the following animal studies: the 18 month mouse carcinogenicity study at 50 mg/kg/day, the 6 month dog study at 36 mg/kg/day, the 6 month hamster study at 40 mg/kg/day, and in acute, high-dose studies in rats and hamsters (50 mg/kg), rabbits (300 mg/kg) and mice (1500 mg/kg). CNS toxicity in the acute high-dose studies was characterized (in mice) by conspicuous vacuolation in the ventral white columns of the spinal cord at a dose of 5000 mg/kg and (in rats) by edema with separation of myelinated fibers of the ventral spinal tracts and sciatic nerve at a dose of 1500 mg/kg. CNS toxicity, characterized by periaxonal vacuolation, was observed in the medulla of dogs that died after treatment for 5 weeks with 48 mg/kg/day; this finding was not observed in the remaining dogs when the dose level was lowered to 36 mg/kg/day. CNS vascular lesions, characterized by perivascular hemorrhages, edema, and mononuclear cell infiltration of perivascular spaces, have been observed in dogs treated with other members of this drug class. No CNS lesions have been observed after chronic treatment for up to 2 years with fluvastatin in the mouse (at doses up to 350 mg/kg/day), rat (up to 24 mg/kg/day), or dog (up to 16 mg/kg/day).
Prominent bilateral posterior Y suture lines in the ocular lens were seen in dogs after treatment with 1, 8, and 16 mg/kg/day for 2 years.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Rhabdomyolysis with myoglobinuria and acute renal failure and myopathy (including myositis) [see Warnings and Precautions (5.1)].
- Liver Enzyme Abnormalities [see Warnings and Precautions (5.2)].
6.1 Clinical Studies Experience in Adult Patients
Because clinical studies on fluvastatin capsules/fluvastatin sodium extended-release tablets are conducted in varying study populations and study designs, the frequency of adverse reactions observed in the clinical studies of fluvastatin capsules/fluvastatin sodium extended-release tablets cannot be directly compared with that in the clinical studies of other statins and may not reflect the frequency of adverse reactions observed in clinical practice.
In the fluvastatin capsule placebo-controlled clinical trials database of 2326 patients treated with fluvastatin1 (age range 18 to 75 years, 44% women, 94% Caucasians, 4% Blacks, 2% other ethnicities) with a median treatment duration of 24 weeks, 3.4% of patients on fluvastatin capsules and 2.3% patients on placebo discontinued due to adverse reactions regardless of causality. The most common adverse reactions that led to treatment discontinuation and occurred at an incidence greater than placebo were: transaminase increased (0.8%), upper abdominal pain (0.3%), dyspepsia (0.3%), fatigue (0.2%) and diarrhea (0.2%).
In the fluvastatin sodium extended-release tablet database of controlled clinical trials of 912 patients treated with fluvastatin sodium extended-release tablets (age range 21 to 87 years, 52% women, 91% Caucasians, 4% Blacks, 5% other ethnicities) with a median treatment duration of 24 weeks, 3.9% of patients on fluvastatin sodium extended-release tablets discontinued due to adverse reactions regardless of causality. The most common adverse reactions that led to treatment discontinuation were abdominal pain (0.7%), diarrhea (0.5%), nausea (0.4%), dyspepsia (0.4%) and chest pain (0.3%).
Clinically relevant adverse experiences occurring in the fluvastatin capsules and fluvastatin sodium extended-release tablets controlled studies with a frequency ≥ 2%, regardless of causality, included the following:
Table 1: Clinical Adverse Events Reported in ≥ 2% in Patients Treated With Fluvastatin Capsules/Fluvastatin Sodium Extended-Release Tablets and at an Incidence Greater Than Placebo in Placebo-Controlled Trials Regardless of Causality (% of Patients) Pooled Dosages - * Controlled trials with fluvastatin capsules (20 and 40 mg daily and 40 mg twice daily) compared to placebo
- † Controlled trials with fluvastatin sodium extended-release tablets, 80 mg as compared to fluvastatin capsules
Fluvastatin Capsules*
Placebo*
Fluvastatin Sodium Extended-Release Tablets†
N = 2326
N = 960
N = 912
(%)
(%)
(%)
Musculoskeletal
Myalgia
5.0
4.5
3.8
Arthritis
2.1
2.0
1.3
Arthropathy
NA
NA
3.2
Respiratory
Sinusitis
2.6
1.9
3.5
Bronchitis
1.8
1.0
2.6
Gastrointestinal
Dyspepsia
7.9
3.2
3.5
Diarrhea
4.9
4.2
3.3
Abdominal pain
4.9
3.8
3.7
Nausea
3.2
2.0
2.5
Flatulence
2.6
2.5
1.4
Tooth disorder
2.1
1.7
1.4
Psychiatric
Insomnia
2.7
1.4
0.8
Genitourinary
Urinary tract infection
1.6
1.1
2.7
Miscellaneous
Headache
8.9
7.8
4.7
Influenza-like symptoms
5.1
5.7
7.1
Accidental Trauma
5.1
4.8
4.2
Fatigue
2.7
2.3
1.6
Allergy
2.3
2.2
1.0
Fluvastatin Capsules Intervention Prevention Study
In the Fluvastatin Capsules Intervention Prevention Study, the effect of fluvastatin 40 mg, administered twice daily on the risk of recurrent cardiac events was assessed in 1677 patients with CHD who had undergone a percutaneous coronary intervention (PCI) procedure. This was a multicenter, randomized, double-blind, placebo-controlled study, patients were treated with dietary/lifestyle counseling and either fluvastatin capsules 40 mg (n = 844) or placebo (n = 833) given twice daily for a median of 3.9 years [see Clinical Studies (14.3)].
Table 2: Clinical Adverse Events Reported in ≥ 2% in Patients Treated With Fluvastatin Capsules/Fluvastatin Sodium Extended-Release Tablets and at an Incidence Greater Than Placebo in the Trial Regardless of Causality (% of Patients) Fluvastatin Capsules
40 mg b.i.d.
Placebo
N = 822
N = 818
(%)
(%)
Cardiac disorders
Atrial fibrillation
2.4
2.0
Gastrointestinal disorders
Abdominal pain upper
6.3
4.5
Constipation
3.3
2.1
Dyspepsia
4.5
4.0
Gastric disorder
2.7
2.1
Nausea
2.7
2.3
General disorders
Fatigue
4.7
3.8
Edema peripheral
4.4
2.9
Infections and infestations
Bronchitis
2.3
2.0
Nasopharyngitis
2.8
2.1
Musculoskeletal and connective tissue disorders
Arthralgia
2.1
1.8
Myalgia
2.2
1.6
Pain in extremity
4.1
2.7
Nervous system disorders
Dizziness
3.9
3.5
Syncope
2.4
2.2
Respiratory disorders
Dyspnea exertional
2.8
2.4
Vascular disorders
Hypertension
5.8
4.2
Intermittent claudication
2.3
2.1
6.2 Clinical Studies Experience in Pediatric Patients
In patients aged < 18 years, efficacy and safety have not been studied for treatment periods longer than two years.
In two open-label, uncontrolled studies, 66 boys and 48 girls with heterozygous familial hypercholesterolemia (9 to 16 years of age, 80% Caucasian, 19% Other [mixed ethnicity], 1% Asians) were treated with fluvastatin sodium administered as fluvastatin capsules 20 mg to 40 mg twice daily, or fluvastatin sodium extended-release tablets, 80 mg [see Clinical Studies (14.2) and Use in Specific Populations (8.4)].
6.3 Postmarketing Experience
Because adverse reactions from spontaneous reports are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following effects have been reported with drugs in this class. Not all the effects listed below have necessarily been associated with fluvastatin sodium therapy.
Musculoskeletal: muscle cramps, myalgia, myopathy, rhabdomyolysis, arthralgias, muscle spasms, muscle weakness, myositis.
There have been rare reports of immune-mediated necrotizing myopathy associated with statin use [see Warnings and Precautions (5.1)].
Neurological: dysfunction of certain cranial nerves (including alteration of taste, impairment of extra-ocular movement, facial paresis), tremor, dizziness, vertigo, paresthesia, hypoesthesia, dysesthesia, peripheral neuropathy, peripheral nerve palsy.
There have been rare postmarketing reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. These cognitive issues have been reported for all statins. The reports are generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
Psychiatric: anxiety, insomnia, depression, psychic disturbances
Respiratory: interstitial lung disease
Hypersensitivity Reactions: An apparent hypersensitivity syndrome has been reported rarely which has included one or more of the following features: anaphylaxis, angioedema, lupus erythematosus-like syndrome, polymyalgia rheumatica, vasculitis, purpura, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR (erythrocyte sedimentation rate) increase, eosinophilia, arthritis, arthralgia, urticaria, asthenia, photosensitivity reaction, fever, chills, flushing, malaise, dyspnea, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome.
Gastrointestinal: pancreatitis, hepatitis, including chronic active hepatitis, cholestatic jaundice, fatty change in liver, cirrhosis, fulminant hepatic necrosis, hepatoma, anorexia, vomiting, fatal and non-fatal hepatic failure.
Skin: rash, dermatitis, including bullous dermatitis, eczema, alopecia, pruritus, a variety of skin changes (e.g., nodules, discoloration, dryness of skin/mucous membranes, changes to hair/nails).
Reproductive: gynecomastia, loss of libido, erectile dysfunction.
Eye: progression of cataracts (lens opacities), ophthalmoplegia.
Laboratory abnormalities: elevated transaminases, alkaline phosphatase, gamma-glutamyl transpeptidase and bilirubin; thyroid function abnormalities.
-
7 DRUG INTERACTIONS
7.1 Cyclosporine
Cyclosporine coadministration increases fluvastatin exposure. Therefore, in patients taking cyclosporine, therapy should be limited to fluvastatin 20 mg twice daily [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.2 Fluconazole
Administration of fluvastatin 40 mg single dose to healthy volunteers pre-treated with fluconazole for 4 days results in an increase of fluvastatin exposure. Therefore, in patients taking fluconazole, therapy should be limited to fluvastatin 20 mg twice daily [see Clinical Pharmacology (12.3)].
7.3 Gemfibrozil
Due to an increased risk of myopathy/rhabdomyolysis when HMG-CoA reductase inhibitors are coadministered with gemfibrozil, concomitant administration of fluvastatin sodium extended-release tablets with gemfibrozil should be avoided.
7.4 Other Fibrates
Because it is known that the risk of myopathy during treatment with HMG-CoA reductase inhibitors is increased with concurrent administration of other fibrates, fluvastatin sodium extended-release tablets should be administered with caution when used concomitantly with other fibrates [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.5 Niacin
The risk of skeletal muscle effects may be enhanced when fluvastatin sodium is used in combination with lipid-modifying doses (≥ 1 g/day) of niacin; a reduction in fluvastatin dosage should be considered in this setting [see Warnings and Precautions (5.1)].
7.6 Glyburide
Concomitant administration of fluvastatin and glyburide increased glyburide exposures. Patients on concomitant therapy of glyburide and fluvastatin should continue to be monitored appropriately [see Clinical Pharmacology (12.3)].
7.7 Phenytoin
Concomitant administration of fluvastatin and phenytoin increased phenytoin exposures. Patients should continue to be monitored appropriately when fluvastatin therapy is initiated or when fluvastatin dose is changed [see Clinical Pharmacology (12.3)].
7.8 Warfarin
Bleeding and/or increased prothrombin times have been reported in patients taking coumarin anticoagulants concomitantly with other HMG-CoA reductase inhibitors. Therefore, patients receiving warfarin-type anticoagulants should have their prothrombin times closely monitored when fluvastatin sodium is initiated or the dosage of fluvastatin sodium is changed.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category X
Fluvastatin sodium extended-release tablets are contraindicated in women who are or may become pregnant [see Contraindications (4)].
Lipid lowering drugs are contraindicated during pregnancy, because cholesterol and cholesterol derivatives are needed for normal fetal development. Serum cholesterol and triglycerides increase during normal pregnancy. Atherosclerosis is a chronic process, and discontinuation of lipid-lowering drugs during pregnancy should have little impact on long-term outcomes of primary hypercholesterolemia therapy.
There are no adequate and well-controlled studies of use with fluvastatin sodium extended-release tablets during pregnancy. Rare reports of congenital anomalies have been received following intrauterine exposure to other statins. In a review2 of about 100 prospectively followed pregnancies in women exposed to other statins, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed the rate expected in the general population. The number of cases is adequate only to exclude a 3 to 4 fold increase in congenital anomalies over background incidence. In 89% of prospectively followed pregnancies, drug treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified.
Teratology studies with fluvastatin in rats and rabbits showed maternal toxicity at high dose levels, but there was no evidence of embryotoxic or teratogenic potential [see Nonclinical Toxicology (13)].
Fluvastatin sodium extended-release tablets should be administered to women of child-bearing potential only when such patients are highly unlikely to conceive and have been informed of the potential hazards. If a woman becomes pregnant while taking fluvastatin sodium extended-release tablets, the drug should be discontinued and the patient advised again as to the potential hazards to the fetus.
8.3 Nursing Mothers
Based on animal data, fluvastatin is present in breast milk in a 2:1 ratio (milk:plasma). Because of the potential for serious adverse reactions in nursing infants, nursing women should not take fluvastatin sodium extended-release tablets [see Contraindications (4)].
8.4 Pediatric Use
The safety and efficacy of fluvastatin sodium extended-release tablets in children and adolescent patients 9 to 16 years of age with heterozygous familial hypercholesterolemia have been evaluated in open-label, uncontrolled clinical trials for a duration of two years. The most common adverse events observed were influenza and infections. In these limited uncontrolled studies, there was no detectable effect on growth or sexual maturation in the adolescent boys or on menstrual cycle length in girls [see Clinical Studies (14.2), Adverse Reactions (6.3) and Dosage and Administration (2.2)]. Adolescent females should be counseled on appropriate contraceptive methods while on fluvastatin sodium therapy [see Contraindications (4)].
8.5 Geriatric Use
Fluvastatin exposures were not significantly different between the nonelderly and elderly populations (age ≥ 65 years) [see Clinical Pharmacology (12.3)]. Since advanced age (≥ 65 years) is a predisposing factor for myopathy, fluvastatin sodium extended-release tablets should be prescribed with caution in the elderly.
8.6 Hepatic Impairment
Fluvastatin sodium extended-release tablets are contraindicated in patients with active liver disease or unexplained, persistent elevations in serum transaminases [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
Dose adjustments for mild to moderate renal impairment are not necessary. Fluvastatin has not been studied at doses greater than 40 mg in patients with severe renal impairment; therefore caution should be exercised when treating such patients at higher doses [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
To date, there has been limited experience with overdosage of fluvastatin. If an overdose occurs, it should be treated symptomatically with laboratory monitoring and supportive measures should be instituted as required. The dialyzability of fluvastatin sodium and of its metabolites in humans is not known at present [see Warnings and Precautions (5)].
In the pediatric population, there have been reports of overdosage with fluvastatin sodium in children including a 2-year-old and the other 3 years of age, either of whom may have possibly ingested fluvastatin sodium. The maximum amount of fluvastatin sodium that could have been ingested was 80 mg (4 x 20 mg capsules). Vomiting was induced by ipecac in both children and no capsules were noted in their emesis. Neither child experienced any adverse symptoms and both recovered from the incident without problems.
In the postmarketing experience there have been reports of accidental ingestion of fluvastatin capsules in infants up to 3 years of age. In one case, increased serum CPK values were noted. There have been reports of intentional overdose in adolescents with the development of hepatic enzyme elevations, convulsions and gastroenteritis/vomiting/diarrhea. One case of intentional overdose as suicide attempt in a 15-year-old female reported ingestion of 2,800 mg fluvastatin sodium extended-release tablets with hepatic enzyme elevation.
-
11 DESCRIPTION
Fluvastatin sodium is a water-soluble cholesterol lowering agent which acts through the inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase.
Fluvastatin sodium is [R*,S*-(E)]-(±)-7-[3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid, monosodium salt. The structural formula is:
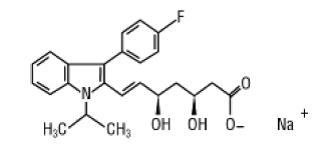
C24H25FNNaO4 M.W. 433.46
This molecular entity is the first entirely synthetic HMG-CoA reductase inhibitor, and is in part structurally distinct from the fungal derivatives of this therapeutic class.
Fluvastatin sodium (hydrated form) is a white to pale yellow, brownish-pale yellow, or reddish-pale yellow hygroscopic powder soluble in water, ethanol and methanol. Fluvastatin sodium extended-release tablets contain fluvastatin sodium (hydrated form), equivalent to 80 mg of fluvastatin, for oral administration.
Active Ingredient: fluvastatin sodium (hydrated form)
Inactive Ingredients: crospovidone, hydroxyethyl cellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol partially hydrolyzed, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fluvastatin sodium is a competitive inhibitor of HMG-CoA reductase, the rate limiting enzyme that converts 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) to mevalonate, a precursor of sterols, including cholesterol. The inhibition of cholesterol biosynthesis reduces the cholesterol in hepatic cells, which stimulates the synthesis of LDL receptors and thereby increases the uptake of LDL particles. The end result of these biochemical processes is a reduction of the plasma cholesterol concentration.
12.3 Pharmacokinetics
Absorption:
Following oral administration of the capsule, fluvastatin reaches peak concentrations in less than 1 hour. The absolute bioavailability is 24% (range 9% to 50%) after administration of a 10 mg dose.
At steady state, administration of fluvastatin with the evening meal results in a 50% decrease in Cmax, an 11% decrease in AUC, and a more than two-fold increase in tmax as compared to administration 4 hours after the evening meal. No significant differences in the lipid-lowering effects were observed between the two administrations. After single or multiple doses above 20 mg, fluvastatin exhibits saturable first-pass metabolism resulting in more than dose proportional plasma fluvastatin concentrations.
Fluvastatin administered as fluvastatin sodium extended-release tablets, 80 mg reaches peak concentration in approximately 3 hours under fasting conditions, after a low-fat meal, or 2.5 hours after a low-fat meal. The mean relative bioavailability of the extended-release tablet is approximately 29% (range: 9% to 66%) compared to that of the fluvastatin immediate-release capsule administered under fasting conditions. Administration of a high-fat meal delayed the absorption (Tmax: 6h) and increased the bioavailability of the extended-release tablet by approximately 50%. However, the maximum concentration of fluvastatin sodium extended-release tablets seen after a high-fat meal is less than the peak concentration following a single dose or twice daily dose of the 40 mg fluvastatin capsule.
Distribution:
Fluvastatin is 98% bound to plasma proteins. The mean volume of distribution (VDss) is estimated at 0.35 L/kg. At therapeutic concentrations, the protein binding of fluvastatin is not affected by warfarin, salicylic acid and glyburide.
Metabolism:
Fluvastatin is metabolized in the liver, primarily via hydroxylation of the indole ring at the 5 and 6 positions. N-dealkylation and beta-oxidation of the side-chain also occurs. The hydroxy metabolites have some pharmacologic activity, but do not circulate in the blood. Fluvastatin has two enantiomers. Both enantiomers of fluvastatin are metabolized in a similar manner.
In vitro data indicate that fluvastatin metabolism involves multiple Cytochrome P450 (CYP) isozymes. CYP2C9 isoenzyme is primarily involved in the metabolism of fluvastatin (approximately 75%), while CYP2C8 and CYP3A4 isoenzymes are involved to a much less extent, i.e., approximately 5% and approximately 20%, respectively.
Excretion:
Following oral administration, fluvastatin is primarily (about 90%) excreted in the feces as metabolites, with less than 2% present as unchanged drug. Approximately 5% of a radiolabeled oral dose were recovered in urine. The elimination half-life (t1/2) of fluvastatin is approximately 3 hours.
Specific Populations
Renal Impairment:
In patients with moderate to severe renal impairment (CLCr 10 to 40 mL/min), AUC and Cmax increased approximately 1.2 fold after administration of a single dose of 40 mg fluvastatin compared to healthy volunteers. In patients with end-stage renal disease on hemodialysis, the AUC increased by approximately 1.5 fold. Fluvastatin sodium extended-release tablets were not evaluated in patients with renal impairment. However, systemic exposures after administration of fluvastatin sodium extended-release tablets are lower than after the 40 mg immediate release capsule.
Hepatic Impairment:
In patients with hepatic impairment due to liver cirrhosis, fluvastatin AUC and Cmax increased approximately 2.5 fold compared to healthy subjects after administration of a single 40 mg dose. The enantiomer ratios of the two isomers of fluvastatin in hepatic impairment patients were comparable to those observed in healthy subjects.
Geriatric:
Plasma levels of fluvastatin are not significantly different in patients age > 65 years compared to patients age 21 to 49 years.
Gender:
In a study evaluating the effect of age and gender on fluvastatin pharmacokinetics, there were no significant differences in fluvastatin exposures between males and females, except between younger females and younger males (both ages 21 to 49 years), where there was an approximate 30% increase in AUC in females. Adjusting for body weight decreases the magnitude of the differences seen. For fluvastatin sodium extended-release tablets, the AUC increases 67% and 77% for women compared to men under fasted and high-fat meal fed conditions, respectively.
Pediatric:
Pharmacokinetic data in the pediatric population are not available.
Drug-Drug Interactions:
Data from drug-drug interactions studies involving coadministration of gemfibrozil, niacin, itraconazole, erythromycin, tolbutamide or clopidogrel indicate that the PK disposition of fluvastatin is not significantly altered when fluvastatin is coadministered with any of these drugs.
The below listed drug interaction information is derived from studies using fluvastatin capsules. Similar studies have not been conducted using the fluvastatin sodium extended-release tablet.
Table 3: Effect of Coadministered Drugs on Fluvastatin Systemic Exposure - * Single dose unless otherwise noted
- † Mean ratio (with/without coadministered drug and no change = 1 fold) or % change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively.
- ‡ Considered clinically significant [see Dosage and Administration (2) and Drug Interactions (7)]
Coadministered drug and dosing regimen
Fluvastatin
Dose (mg)*
Change in AUC†
Change in Cmax†
Cyclosporine – stable dose (b.i.d.)‡
20 mg QD for 14 weeks
↑ 90%
↑ 30%
Fluconazole 400 mg QD day 1,200 mg b.i.d. day 2 to 4‡
40 mg QD
↑ 84%
↑ 44%
Cholestyramine 8 g QD
20 mg QD administered 4 hrs after a meal plus cholestyramine
↓ 51%
↓ 83%
Rifampicin 600 mg QD for 6 days
20 mg QD
↓ 53%
↓ 42%
Cimetidine 400 mg b.i.d. for 5 days, QD on Day 6
20 mg QD
↑ 30%
↑ 40%
Ranitidine 150 mg b.i.d. for 5 days, QD on Day 6
20 mg QD
↑ 10%
↑ 50%
Omeprazole 40 mg QD for 6 days
20 mg QD
↑ 20%
↑ 37%
Phenytoin 300 mg QD
40 mg b.i.d. for 5 days
↑ 40%
↑ 27%
Propranolol 40 mg b.i.d. for 3.5 days
40 mg QD
↓ 5%
No change
Digoxin 0.1 to 0.5 mg QD for 3 weeks
40 mg QD
No change
↑ 11%
Diclofenac 25 mg QD
40 mg QD for 8 days
↑ 50%
↑ 80%
Glyburide 5 to 20 mg QD for 22 days
40 mg b.i.d. for 14 days
↑ 51%
↑ 44%
Warfarin 30 mg QD
40 mg QD for 8 days
↑ 30%
↑ 67%
Clopidogrel 300 mg loading dose on day 10, 75 mg QD on days 11 to 19
80 mg XL QD for 19 days
↓ 2%
↑ 27%
Data from drug-drug interaction studies involving fluvastatin and coadministration of either gemfibrozil, tolbutamide or losartan indicate that the PK disposition of either gemfibrozil, tolbutamide or losartan is not significantly altered when coadministered with fluvastatin.
Table 4: Effect of Fluvastatin Coadministration on Systemic Exposure of Other Drugs - * Single dose unless otherwise noted
- † Mean ratio (with/without coadministered drug and no change = 1 fold) or % change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively.
- ‡ Considered clinically significant [see Dosage and Administration (2) and Drug Interactions (7)]
Fluvastatin dosage regimen
Coadministered drug
Name and Dose (mg)*
Change in AUC†
Change in Cmax†
40 mg QD for 5 days
Phenytoin 300 mg QD‡
↑ 20%
↑ 5%
40 mg b.i.d. for 21 days
Glyburide 5 to 20 mg QD for 22 days‡
↑ 70%
↑ 50%
40 mg QD for 8 days
Diclofenac 25 mg QD
↑ 25%
↑ 60%
40 mg QD for 8 days
Warfarin 30 mg QD
S-warfarin: ↑ 7%
S-warfarin: ↑ 10%
R-warfarin: no change
R-warfarin: ↑ 6%
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2 year study was performed in rats at dose levels of 6, 9, and 18 to 24 (escalated after 1 year) mg/kg/day. These treatment levels represented plasma drug levels of approximately 9, 13, and 26 to 35 times the mean human plasma drug concentration after a 40 mg oral dose. A low incidence of forestomach squamous papillomas and 1 carcinoma of the forestomach at the 24 mg/kg/day dose level was considered to reflect the prolonged hyperplasia induced by direct contact exposure to fluvastatin sodium rather than to a systemic effect of the drug. In addition, an increased incidence of thyroid follicular cell adenomas and carcinomas was recorded for males treated with 18 to 24 mg/kg/day. The increased incidence of thyroid follicular cell neoplasm in male rats with fluvastatin sodium appears to be consistent with findings from other HMG-CoA reductase inhibitors. In contrast to other HMG-CoA reductase inhibitors, no hepatic adenomas or carcinomas were observed.
The carcinogenicity study conducted in mice at dose levels of 0.3, 15 and 30 mg/kg/day revealed, as in rats, a statistically significant increase in forestomach squamous cell papillomas in males and females at 30 mg/kg/day and in females at 15 mg/kg/day. These treatment levels represented plasma drug levels of approximately 0.05, 2, and 7 times the mean human plasma drug concentration after a 40 mg oral dose.
No evidence of mutagenicity was observed in vitro, with or without rat-liver metabolic activation, in the following studies: microbial mutagen tests using mutant strains of Salmonella typhimurium or Escherichia coli; malignant transformation assay in BALB/3T3 cells; unscheduled DNA synthesis in rat primary hepatocytes; chromosomal aberrations in V79 Chinese Hamster cells; HGPRT V79 Chinese Hamster cells. In addition, there was no evidence of mutagenicity in vivo in either a rat or mouse micronucleus test.
In a study in rats at dose levels for females of 0.6, 2 and 6 mg/kg/day and at dose levels for males of 2, 10 and 20 mg/kg/day, fluvastatin sodium had no adverse effects on the fertility or reproductive performance.
Seminal vesicles and testes were small in hamsters treated for 3 months at 20 mg/kg/day (approximately three times the 40 mg human daily dose based on surface area, mg/m2). There was tubular degeneration and aspermatogenesis in testes as well as vesiculitis of seminal vesicles. Vesiculitis of seminal vesicles and edema of the testes were also seen in rats treated for 2 years at 18 mg/kg/day (approximately 4 times the human Cmax achieved with a 40 mg daily dose).
Fluvastatin sodium produced delays in skeletal development in rats at doses of 12 mg/kg/day and in rabbits at doses of 10 mg/kg/day. Malaligned thoracic vertebrae were seen in rats at 36 mg/kg, a dose that produced maternal toxicity. These doses resulted in 2 times (rat at 12 mg/kg) or 5 times (rabbit at 10 mg/kg) the 40 mg human exposure based on mg/m2 surface area. A study in which female rats were dosed during the third trimester at 12 and 24 mg/kg/day resulted in maternal mortality at or near term and postpartum. In addition, fetal and neonatal lethality were apparent. No effects on the dam or fetus occurred at 2 mg/kg/day. A second study at levels of 2, 6, 12 and 24 mg/kg/day confirmed the findings in the first study with neonatal mortality beginning at 6 mg/kg. A modified Segment III study was performed at dose levels of 12 or 24 mg/kg/day with or without the presence of concurrent supplementation with mevalonic acid, a product of HMG-CoA reductase which is essential for cholesterol biosynthesis. The concurrent administration of mevalonic acid completely prevented the maternal and neonatal mortality but did not prevent low body weights in pups at 24 mg/kg on days 0 and 7 postpartum.
-
14 CLINICAL STUDIES
14.1 Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
Fluvastatin sodium extended-release tablets have been studied in five controlled studies of patients with primary hypercholesterolemia and mixed dyslipidemia. Fluvastatin sodium extended-release tablets were administered to over 900 patients in trials from 4 to 26 weeks in duration. In the three largest of these studies, fluvastatin sodium extended-release tablets given as a single daily dose of 80 mg significantly reduced Total-C, LDL-C, TG and Apo B and resulted in increases in HDL-C (Table 5).
In patients with primary mixed dyslipidemia as defined by baseline plasma TG levels ≥ 200 mg/dL and < 400 mg/dL, treatment with fluvastatin sodium extended-release tablets produced significant decreases in Total-C, LDL-C, TG and Apo B and variable increases in HDL-C (Table 5).
Table 5: Median Percent Change in Lipid Parameters From Baseline to Week 24 Endpoint All Active Controlled Trials (Fluvastatin Sodium Extended-Release Tablets) - * Data for fluvastatin sodium extended-release tablet, 80 mg from three 24 week controlled trials
Total Chol
TG
LDL
Apo B
HDL
Dose
N
% ∆
N
% ∆
N
% ∆
N
% ∆
N
% ∆
All Patients
Fluvastatin sodium extended-release tablets, 80 mg*
750
-25
750
-19
748
-35
745
-27
750
+7
Baseline TG ≥ 200 mg/dL
Fluvastatin sodium extended-release tablets, 80 mg*
239
-25
239
-25
237
-33
235
-27
239
+11
14.2 Heterozygous Familial Hypercholesterolemia in Pediatric Patients
Fluvastatin sodium was studied in two open-label, uncontrolled, dose-titration studies. The first study enrolled 29 pre-pubertal boys, 9 to 12 years of age, who had an LDL-C level > 90th percentile for age and one parent with primary hypercholesterolemia and either a family history of premature ischemic heart disease or tendon xanthomas. The mean baseline LDL-C was 226 mg/dL (range: 137 to 354 mg/dL). All patients were started on fluvastatin capsules 20 mg daily with dose adjustments every 6 weeks to 40 mg daily then 80 mg daily (40 mg b.i.d.) to achieve an LDL-C goal between 96.7 to 123.7 mg/dL. Endpoint analyses were performed at Year 2. Fluvastatin sodium decreased plasma levels of Total-C and LDL-C by 21% and 27%, respectively. The mean achieved LDL-C was 161 mg/dL (range: 74 to 336 mg/dL).
The second study enrolled 85 male and female patients, 10 to 16 years of age, who had an LDL-C > 190 mg/dL or LDL-C > 160 mg/dL and one or more risk factors for coronary heart disease, or LDL-C > 160 mg/dL and a proven LDL-receptor defect. The mean baseline LDL-C was 225 mg/dL (range: 148 to 343 mg/dL). All patients were started on fluvastatin capsules 20 mg daily with dose adjustments every 6 weeks to 40 mg daily then 80 mg daily (fluvastatin sodium extended release tablet, 80 mg) to achieve an LDL-C goal of < 130 mg/dL. Endpoint analyses were performed at Week 114. Fluvastatin sodium decreased plasma levels of Total-C and LDL-C by 22% and 28%, respectively. The mean achieved LDL-C was 159 mg/dL (range: 90 to 295 mg/dL).
The majority of patients in both studies (83% in the first study and 89% in the second study) were titrated to the maximum daily dose of 80 mg. At study endpoint, 26% to 30% of patients in both studies achieved a targeted LDL-C goal of < 130 mg/dL. The long-term efficacy of fluvastatin sodium therapy in childhood to reduce morbidity and mortality in adulthood has not been established.
14.3 Secondary Prevention of Cardiovascular Disease
In the Fluvastatin Capsules Intervention Prevention Study, the effect of fluvastatin capsules 40 mg administered twice daily on the risk of recurrent cardiac events (time to first occurrence of cardiac death, nonfatal myocardial infarction, or revascularization) was assessed in 1677 patients with CHD who had undergone a percutaneous coronary intervention (PCI) procedure (mean time from PCI to randomization = 3 days). In this multicenter, randomized, double-blind, placebo-controlled study, patients were treated with dietary/lifestyle counseling and either fluvastatin 40 mg (n = 844) or placebo (n = 833) given twice daily for a median of 3.9 years. The study population was 84% male, 98% Caucasian, with 37% > 65 years of age. Mean baseline lipid concentrations were: total cholesterol 201 mg/dL, LDL-C 132 mg/dL, triglycerides 70 mg/dL and HDL-C 39 mg/dL.
Fluvastatin capsules significantly reduced the risk of recurrent cardiac events (Figure 1) by 22% (p = 0.013, 181 patients in the fluvastatin capsules group vs. 222 patients in the placebo group). Revascularization procedures comprised the majority of the initial recurrent cardiac events (143 revascularization procedures in the fluvastatin capsules group and 171 in the placebo group). Consistent trends in risk reduction were observed in patients > 65 years of age.
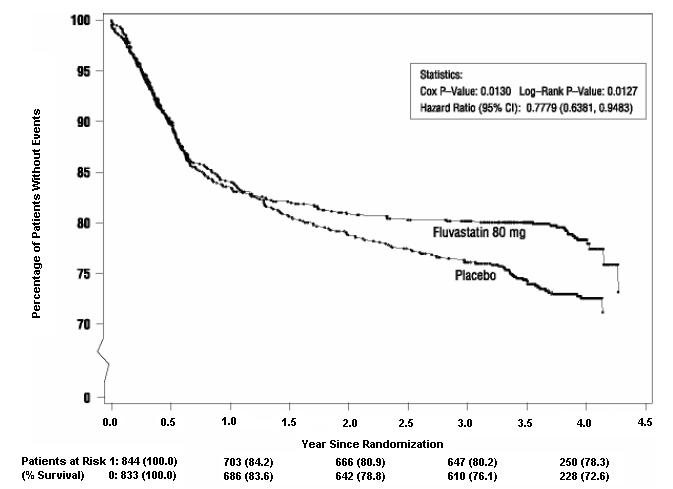
Figure 1: Primary Endpoint – Recurrent Cardiac Events (Cardiac Death, Nonfatal MI or Revascularization Procedure) (ITT Population)
Outcome data for the Fluvastatin Capsules Intervention Prevention Study are shown in Figure 2. After exclusion of revascularization procedures (CABG and repeat PCI) occurring within the first 6 months of the initial procedure involving the originally instrumental site, treatment with fluvastatin capsules was associated with a 32% (p = 0.002) reduction in risk of late revascularization procedures (CABG or PCI occurring at the original site > 6 months after the initial procedure, or at another site).
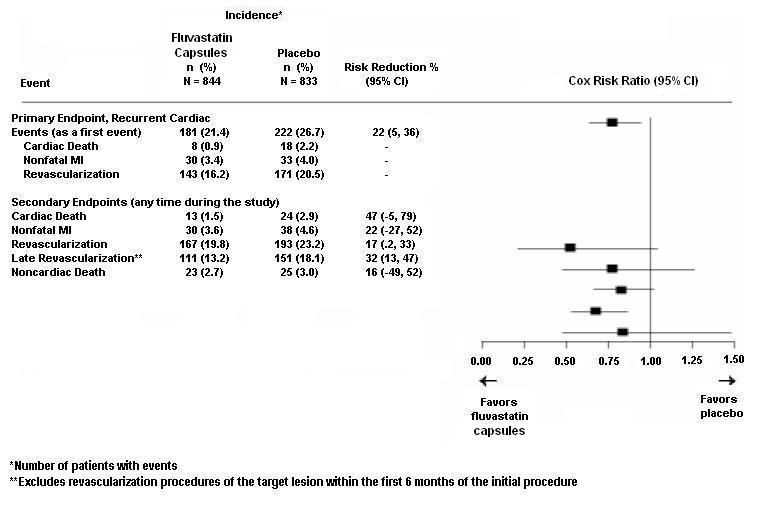
Figure 2: Fluvastatin Capsules Intervention Prevention Study - Primary and Secondary Endpoints
In the Lipoprotein and Coronary Atherosclerosis Study (LCAS), the effect of fluvastatin capsule therapy on coronary atherosclerosis was assessed by quantitative coronary angiography (QCA) in patients with CAD and mild to moderate hypercholesterolemia (baseline LDL-C range 115 to 190 mg/dL). In this randomized double-blind, placebo-controlled trial, 429 patients were treated with conventional measures (Step 1 AHA Diet) and either fluvastatin 40 mg/day or placebo. In order to provide treatment to patients receiving placebo with LDL-C levels ≥ 160 mg/dL at baseline, adjunctive therapy with cholestyramine was added after Week 12 to all patients in the study with baseline LDL-C values of ≥ 160 mg/dL which were present in 25% of the study population. Quantitative coronary angiograms were evaluated at baseline and 2.5 years in 340 (79%) angiographic evaluable patients.
Compared to placebo, fluvastatin capsules significantly slowed the progression of coronary atherosclerosis as measured by within-patient per-lesion change in minimum lumen diameter (MLD), the primary endpoint (Figure 3 below), percent diameter stenosis (Figure 4), and the formation of new lesions (13% of all fluvastatin patients versus 22% of all placebo patients). A significant difference in favor of fluvastatin capsules was found between all fluvastatin and all placebo patients in the distribution among the three categories of definite progression, definite regression, and mixed or no change. Beneficial angiographic results (change in MLD) were independent of patients’ gender and consistent across a range of baseline LDL-C levels.
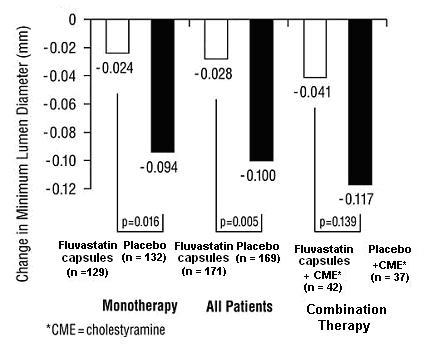
Figure 3: Change in Minimum Lumen Diameter (mm)
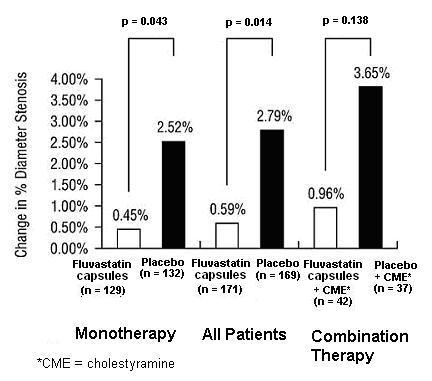
Figure 4: Change in % Diameter Stenosis
-
15 REFERENCES
1. National Cholesterol Education Program (NCEP): Highlights of the Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics. 89(3):495-501.1992.
2. Manson, J.M., Freyssinges, C., Ducrocq, M.B., Stephenson, W.P., Postmarketing Surveillance of Lovastatin and Simvastatin Exposure During Pregnancy, Reproductive Toxicology, 10(6): 439-446, 1996.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Fluvastatin sodium extended-release tablets are available as:
80 mg - white to off-white, film-coated, round tablets, debossed with “TV” on one side of the tablet and “7446” on the other side of the tablet, in bottles of 30 and 100. (NDC: 0093-7446-56, NDC: 0093-7446-01)
-
17 PATIENT COUNSELING INFORMATION
Information for Patients
Patients taking fluvastatin sodium extended-release tablets should be advised that high cholesterol is a chronic condition and they should adhere to their medication along with their National Cholesterol Education Program (NCEP)-recommended diet, a regular exercise program, and periodic testing of a fasting lipid panel to determine goal attainment.
Patients should be advised about substances they should not take concomitantly with fluvastatin sodium extended-release tablets [see Warnings and Precautions (5.1)]. Patients should also be advised to inform other healthcare professionals prescribing a new medication that they are taking fluvastatin sodium extended-release tablets.
17.1 Muscle Pain
Patients starting therapy with fluvastatin sodium extended-release tablets should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever or if these muscle signs or symptoms persist after discontinuing fluvastatin sodium extended-release tablets.
17.2 Liver Enzymes
It is recommended that liver enzyme tests be performed before the initiation of fluvastatin sodium extended-release tablets and if signs or symptoms of liver injury occur. All patients treated with fluvastatin sodium extended-release tablets should be advised to report promptly any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
17.3 Pregnancy
Women of childbearing age should be advised to use an effective method of birth control to prevent pregnancy while using fluvastatin sodium extended-release tablets. Discuss future pregnancy plans with your patients, and discuss when to stop taking fluvastatin sodium extended-release tablets if they are trying to conceive. Patients should be advised that if they become pregnant they should stop taking fluvastatin sodium extended-release tablets and call their healthcare professional.
17.4 Breastfeeding
Women who are breastfeeding should not use fluvastatin sodium extended-release tablets. Patients who have a lipid disorder and are breastfeeding should be advised to discuss the options with their healthcare professional.
Manufactured In Israel By:
Teva Pharmaceutical Ind. Ltd.
Jerusalem, 9777402, Israel
Manufactured For:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Rev. C 8/2017
-
PATIENT INFORMATION
FDA-Approved Patient Labeling
Fluvastatin (floo'' va stat' in) Sodium Extended-Release Tablets, 80 mg
You must read and follow all instructions before using fluvastatin sodium extended-release tablets.
Read the Patient Information every time you or a family member gets fluvastatin sodium extended-release tablets. There may be new information. This Patient Information does not take the place of talking with your doctor about your medical condition or treatment. If you have any questions about fluvastatin sodium extended-release tablets, ask your doctor or pharmacist.
What are fluvastatin sodium extended-release tablets?
Fluvastatin sodium extended-release tablets are prescription medicines called "statins" that lower cholesterol in your blood. They lower the "bad" cholesterol and triglycerides in your blood. They can raise your "good" cholesterol as well.
Fluvastatin sodium extended-release tablets are for people whose cholesterol does not come down enough with exercise and a low-fat diet alone.
Fluvastatin sodium extended-release tablets may be used in patients with heart disease (coronary artery disease) to:
- lower the chances of heart problems which would require procedures to help restore blood flow to the heart.
- slow the buildup of too much cholesterol in the arteries of the heart.
Treatment with fluvastatin sodium extended-release tablets has not been shown to prevent heart attacks or stroke.
Fluvastatin sodium extended-release tablets are only taken one time a day.
Who should not take fluvastatin sodium extended-release tablets?
Do not take fluvastatin sodium extended-release tablets if you:
- are pregnant or think you may be pregnant, or are planning to become pregnant. Fluvastatin sodium extended-release tablets may harm your unborn baby. If you get pregnant, stop taking fluvastatin sodium extended-release tablets and call your doctor right away.
- are breast-feeding. Fluvastatin sodium can pass into your breast milk and may harm your baby
- have liver problems
- are allergic to fluvastatin sodium or any inactive ingredients of the tablet. The active ingredient in fluvastatin sodium extended-release tablets is fluvastatin. See the end of this leaflet for a complete list of ingredients in fluvastatin sodium extended-release tablets.
Fluvastatin sodium extended-release tablets have not been studied in children under 9 years of age.
Before taking fluvastatin sodium extended-release tablets, tell your doctor if you:
- have muscle aches or weakness
- drink more than 2 glasses of alcohol daily
- have diabetes
- have a thyroid problem
- have kidney problems
Some medicines should not be taken with fluvastatin sodium extended-release tablets. Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Fluvastatin sodium extended-release tablets and certain other medicines can interact causing serious side effects. Especially tell your doctor if you take medicines for:
- your immune system
- cholesterol
- infections
- heart failure
- seizures
- diabetes
- heartburn or stomach ulcers
Know all the medicines you take. Keep a list of all the medicines you take with you to show your doctor and pharmacist.
How should I take fluvastatin sodium extended-release tablets?
- Your doctor will prescribe the medicine that is right for you. Take fluvastatin sodium extended-release tablets exactly as prescribed. Do not change your dose or stop fluvastatin sodium extended-release tablets without talking to your doctor. Your doctor may do blood tests to check your cholesterol levels during treatment with fluvastatin sodium extended-release tablets. Your dose of fluvastatin sodium extended-release tablets may be changed based on these blood test results.
- Fluvastatin sodium extended-release tablets may be taken at any time of the day. Fluvastatin sodium extended-release tablets can be taken with or without food.
- Fluvastatin sodium extended-release tablets must be swallowed whole with a liquid. Do not break, crush or chew fluvastatin sodium extended-release tablets. Tell your doctor if you cannot swallow tablets whole. You may need fluvastatin capsules or a different medicine instead of fluvastatin sodium extended-release tablets.
- Your doctor should start you on a low-fat and low-cholesterol diet before giving you fluvastatin sodium extended-release tablets. Stay on this low-fat and low-cholesterol diet while taking fluvastatin sodium extended-release tablets.
- If you miss a dose of fluvastatin sodium extended-release tablets, take it as soon as you remember. Do not take fluvastatin sodium extended-release tablets if it has been more than 12 hours since your last dose. Wait and take the next dose at your regular time. Do not take 2 doses of fluvastatin sodium extended-release tablets at the same time.
- If you take too many fluvastatin sodium extended-release tablets or overdose, call your doctor or Poison Control Center right away. Or, go to the nearest emergency room.
What should I avoid while taking fluvastatin sodium extended-release tablets?
- Talk to your doctor before you start any new medicines. This includes prescription and non-prescription medicines, vitamins and herbal supplements. Fluvastatin sodium extended-release tablets and certain other medicines can interact causing serious side effects.
- Do not get pregnant. If you get pregnant, stop taking fluvastatin sodium extended-release tablets right away and call your doctor.
What are the possible side effects of fluvastatin sodium extended-release tablets?
When taking fluvastatin sodium extended-release tablets, some patients may develop serious side effects, including:
muscle problems. Call your health care professional right away if you experience unexplained muscle pain, tenderness, or weakness especially with fever. This may be an early sign of a rare muscle problem that could lead to serious kidney problems.
The risk of muscle problems is greater in people who are 65 years of age or older, or who already have thyroid or kidney problems. The chance of muscle problems may be increased if you are taking certain other medicines with fluvastatin sodium extended-release tablets.
If you have muscle problems that do not go away even after your health care professional has advised you to stop taking fluvastatin sodium extended-release tablets, notify your health care professional. Your health care professional may do further tests to diagnose the cause of your muscle problems.
liver problems. Your doctor should do blood tests to check your liver before you start taking fluvastatin sodium extended-release tablets, and if you have symptoms of liver problems while you take fluvastatin sodium extended-release tablets. Call your doctor right away if you have the following symptoms of liver problems:
- feel tired or weak
- loss of appetite
- upper belly pain
- dark amber colored urine
- yellowing of your skin or the whites of your eyes
The most common side effects of fluvastatin sodium extended-release tablets are headache, upset stomach and stomach pain, diarrhea, flu-like symptoms, muscle pain, sinus infection, tiredness, or trouble sleeping. These side effects are usually mild and may go away. The following additional side effects have been reported with fluvastatin sodium extended-release tablets: memory loss, and confusion.
Talk to your doctor or pharmacist if you have side effects that bother you or that will not go away.
These are not all the side effects of fluvastatin sodium extended-release tablets. Ask your doctor or pharmacist for a complete list.
How should I store fluvastatin sodium extended-release tablets?
- Store fluvastatin sodium extended-release tablets at room temperature, 20° to 25°C (68° to 77°F). Protect from light.
- Do not keep medicine that is out of date or that you no longer need.
- Keep fluvastatin sodium extended-release tablets out of the reach of children. Be sure that if you throw medicines away, they are out of the reach of children.
General information about fluvastatin sodium extended-release tablets
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use fluvastatin sodium extended-release tablets for a condition for which they were not prescribed. Do not give fluvastatin sodium extended-release tablets to other people, even if they have the same problem you have; they may harm them.
For more information, call 1-888-838-2872.
What are the ingredients in fluvastatin sodium extended-release tablets?
Active Ingredient: fluvastatin sodium (hydrated form)
Inactive Ingredients: crospovidone, hydroxyethyl cellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol partially hydrolyzed, talc, and titanium dioxide.
Manufactured In Israel By:
Teva Pharmaceutical Ind. Ltd.
Jerusalem, 9777402, Israel
Manufactured For:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Rev. B 8/2017
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
FLUVASTATIN SODIUM
fluvastatin sodium tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0093-7446 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUVASTATIN SODIUM (UNII: PYF7O1FV7F) (FLUVASTATIN - UNII:4L066368AS) FLUVASTATIN 80 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) HYDROXYETHYL CELLULOSE (5500 MPA.S AT 2%) (UNII: M825OX60H9) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 10mm Flavor Imprint Code TV;7446 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0093-7446-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/02/2016 2 NDC: 0093-7446-56 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/02/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079011 06/02/2016 Labeler - Teva Pharmaceuticals USA, Inc. (001627975)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.