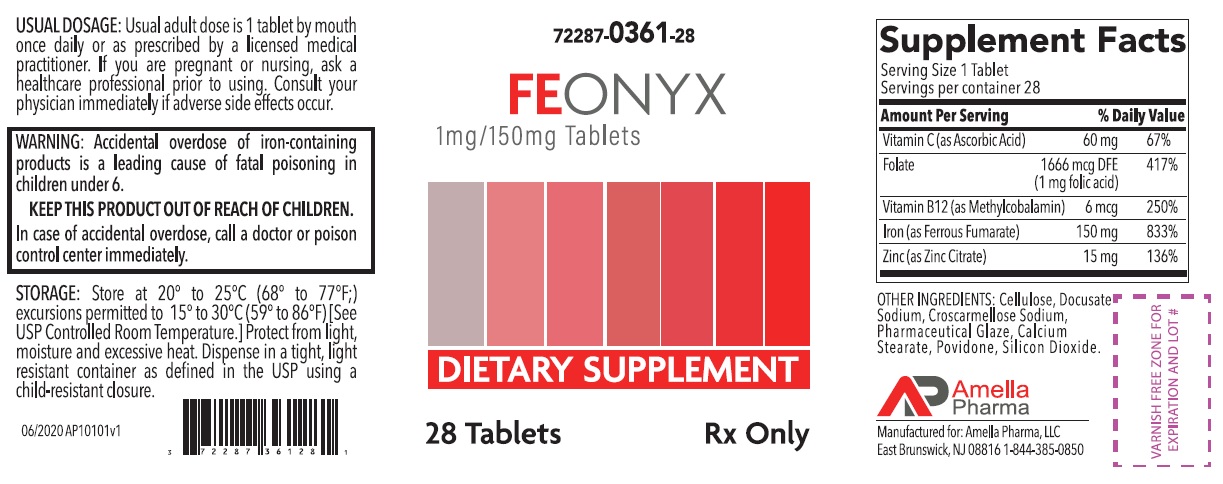
FEONYX- ascorbic acid, folic acid, methylcobalamin, ferrous fumarate, zinc citrate tablet, coated
FEONYX by
Drug Labeling and Warnings
FEONYX by is a Other medication manufactured, distributed, or labeled by Amella Pharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HEALTH CLAIM:FEONYX Tablets Dietary Supplement with Iron
Dispensed by Prescription†
Supplements Facts Serving Size: 1 Tablet
Servings per container: 28
Amount per
Serving
% Daily
Value
Vitamin C (as Ascorbic Acid) 60 mg 67% Folate (1 mg Folic Acid) 1666 mcg DFE 417% Vitamin B12 (as Methylcobalamin) 6 mcg 250% Iron (as Ferrous Fumarate)
(Equivalent to about 50mg of Elemental Iron)
150 mg
833% Zinc (as Zinc Citrate) 15 mg 136% OTHER INGREDIENTS: Cellulose, Docusate Sodium, Croscarmellose Sodium, Pharmaceutical Glaze, Calcium Stearate, Povidone, Silicon Dioxide
-
DESCRIPTION
FEONYX Tablets is a professionally prescribed orally administrated hematinic multivitamin/multimineral dietary supplement used to improve the nutritional status of patients with iron deficiency; this includes women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and non-lactating mothers. THIS PRODUCT IS NOT INDICATED FOR USE IN CHILDREN.
FEONYX Tablets are small, round, dark brown, clear-coated tablet, with debossed "A" on one side.
-
CONTRAINDICATIONS
FEONYX should not be used by patients with a known hypersensitivity to any of the listed ingredients. All iron compounds are contraindicated in patients with hemochromatosis, hemosiderosis, or hemolytic anemias.
PRECAUTIONS:
General: Take 2 hours after meals. Do not exceed recommended dose. Discontinue use if symptoms of intolerance appear. The type of anemia and the underlying cause or causes should be determined before starting therapy with FEONYX tablets. Ensure Hgb, Hct, Reticulocyte count are determined before starting therapy and periodically thereafter during prolonged treatment. Periodically review therapy to determine if it needs to be continued without change or if a dose change is indicated. Since the anemia may be a result of a systemic disturbance, such as recurrent blood loss, the underlying cause or causes should be corrected, if possible.
Folic Acid: Folic acid alone is an improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia assessment, such that hematologic remission can occur while neurological manifestations remain progressive. Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
Pediatric Use: Safety and effectiveness in pediatric population have not been established.
Geriatric Use: Safety and effectiveness in elderly population have not been established.
KEEP OUT OF REACH OF CHILDREN.
DRUG INTERACTIONS
Prescriber should be aware of a number of iron/drug interactions, including antacids, tetracyclines, or fluoroquinolones
ADVERSE REACTIONS
Adverse reactions with iron therapy may include GI irritation, constipation, diarrhea, nausea, vomiting, and dark stools. Adverse reactions with iron therapy are usually transient. Allergic sensitization has been reported following both oral and parental administration of folic acid.
OVERDOSAGE
The clinical course of acute iron overdosage can be variable. Symptoms may include abdominal pain, metabolic acidosis, anuria, CNS damage, coma, convulsions, death, dehydration, diffuse vascular congestion, hepatic cirrhosis, hypotension, hypothermia, lethargy, nausea, vomiting, diarrhea, tarry stools, melena, hematemesis, hypotension, tachycardia, hyperglycemia, dehydration, drowsiness, pallor, cyanosis, lassitude, seizures, and shock.
-
WARNINGS
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient.
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. - DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
FEONYX Tablets are small, round, dark brown, clear-coated tablet, with debossed "A" on one side.
FEONYX Tablets is available as the following:
72287-361-28* 28ct bottleSTORAGE
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Protect from light, moisture and avoid excessive heat. Dispense in a tight, light resistant container as defined by the USP using a child-resistant closure.
Manufactured for:
Amella Pharma, LLC
E Brunswick, NJ 08816Call your doctor for medical advice about side effects. You may report side effects to Amella Pharma, LLC at 1-844-385-0850.
Issued: 06/2020 AP10102v1
*Amella Pharma does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
† This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels increased risk associated with masking of B12 deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (61 FR 8760). The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription (Rx). This is not an Orange Book product. This product may be administered only under a physician’s supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
1. Federal Register Notice of August 2, 1973 (38 FR 20750)
2. Federal Register Notice of October 17, 1980 (45 FR 69043, 69044)
3. Federal Register Notice of March 5, 1996 (61 FR 8760) - Packaging
-
INGREDIENTS AND APPEARANCE
FEONYX
ascorbic acid, folic acid, methylcobalamin, ferrous fumarate, zinc citrate tablet, coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:72287-361 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 60 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg METHYLCOBALAMIN (UNII: BR1SN1JS2W) (METHYLCOBALAMIN - UNII:BR1SN1JS2W) METHYLCOBALAMIN 6 ug FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 150 mg ZINC CITRATE (UNII: K72I3DEX9B) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 15 mg Inactive Ingredients Ingredient Name Strength POWDERED CELLULOSE (UNII: SMD1X3XO9M) DOCUSATE SODIUM (UNII: F05Q2T2JA0) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SHELLAC (UNII: 46N107B71O) CALCIUM STEARATE (UNII: 776XM7047L) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:72287-361-28 28 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 11/18/2020 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 11 mm scoring 1 imprint Labeler - Amella Pharma, LLC (081189492)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.