BESPONSA- inotuzumab ozogamicin injection, powder, lyophilized, for solution
Besponsa by
Drug Labeling and Warnings
Besponsa by is a Prescription medication manufactured, distributed, or labeled by Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc., Pharmacia and Upjohn Company LLC, Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC, Wyeth Pharmaceutical Division of Wyeth Holdings LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BESPONSA™ safely and effectively. See full prescribing information for BESPONSA.
BESPONSA (inotuzumab ozogamicin) for injection, for intravenous use
Initial U.S. Approval: 2017WARNING: HEPATOTOXICITY, INCLUDING HEPATIC VENO-OCCLUSIVE DISEASE (VOD) (ALSO KNOWN AS SINUSOIDAL OBSTRUCTION SYNDROME and INCREASED RISK OF POST- HEMATOPOIETIC STEM CELL TRANSPLANT (HSCT) NON-RELAPSE MORTALITY
See full prescribing information for complete boxed warning.
INDICATIONS AND USAGE
BESPONSA is a CD22-directed antibody-drug conjugate (ADC) indicated for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). (1)
DOSAGE AND ADMINISTRATION
- Pre-medicate with a corticosteroid, antipyretic, and antihistamine prior to all infusions. (2.2)
- Dosing regimens for Cycle 1 and subsequent cycles, depending on the response to treatment, are shown below. See full prescribing information for dosing details. (2)
Day 1 Day 8 Day 15 - * For patients who achieve a CR or a CRi, and/or to allow for recovery from toxicity, the cycle length may be extended up to 28 days (i.e., 7-day treatment-free interval starting on Day 21).
Dosing regimen for Cycle 1 All patients: Dose 0.8 mg/m2 0.5 mg/m2 0.5 mg/m2 Cycle length 21 days* Dosing regimen for subsequent cycles depending on response to treatment Patients who have achieved a CR or CRi: Dose 0.5 mg/m2 0.5 mg/m2 0.5 mg/m2 Cycle length 28 days Patients who have not achieved a CR or CRi: Dose 0.8 mg/m2 0.5 mg/m2 0.5 mg/m2 Cycle length 28 days - See full prescribing information for instructions on reconstitution of lyophilized powder, and preparation and administration of reconstituted drug. (2.4)
DOSAGE FORMS AND STRENGTHS
For injection: 0.9 mg lyophilized powder in a single-dose vial for reconstitution and further dilution. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Myelosuppression: Monitor complete blood counts; for signs and symptoms of infection; bleeding/hemorrhage; or other effects of myelosuppression during treatment; manage appropriately. (5.3)
- Infusion related reactions: Monitor for infusion related reactions during and for at least 1 hour after infusion ends. (5.4)
- QT interval prolongation: Obtain electrocardiograms (ECGs) and electrolytes at baseline and monitor during treatment. Monitor more frequently when using concomitant mediations known to prolong QT interval. (5.5)
- Embryo-fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.6)
ADVERSE REACTIONS
The most common (≥ 20%) adverse reactions are thrombocytopenia, neutropenia, infection, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, gamma-glutamyltransferase increased, and hyperbilirubinemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: HEPATOTOXICITY, INCLUDING HEPATIC VENO-OCCLUSIVE DISEASE (VOD) (ALSO KNOWN AS SINUSOIDAL OBSTRUCTION SYNDROME and INCREASED RISK OF POST-HEMATOPOIETIC STEM CELL TRANSPLANT (HSCT) NON-RELAPSE MORTALITY
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Recommended Pre-medications and Cytoreduction
2.3 Dose Modification
2.4 Instructions for Reconstitution, Dilution, and Administration
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity, Including Hepatic Veno-occlusive Disease (VOD) (also known as Sinusoidal Obstruction Syndrome)
5.2 Increased Risk of Post-Transplant Non-Relapse Mortality
5.3 Myelosuppression
5.4 Infusion Related Reactions
5.5 QT Interval Prolongation
5.6 Embryo-Fetal Toxicity
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
7. DRUG INTERACTIONS
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14. CLINICAL STUDIES
15. REFERENCES
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17. PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: HEPATOTOXICITY, INCLUDING HEPATIC VENO-OCCLUSIVE DISEASE (VOD) (ALSO KNOWN AS SINUSOIDAL OBSTRUCTION SYNDROME and INCREASED RISK OF POST-HEMATOPOIETIC STEM CELL TRANSPLANT (HSCT) NON-RELAPSE MORTALITY
HEPATOTOXICITY, INCLUDING VOD
- Hepatotoxicity, including fatal and life-threatening VOD occurred in patients with relapsed or refractory acute lymphoblastic leukemia (ALL) who received BESPONSA. The risk of VOD was greater in patients who underwent HSCT after BESPONSA treatment; use of HSCT conditioning regimens containing 2 alkylating agents and last total bilirubin level ≥ upper limit of normal (ULN) before HSCT were significantly associated with an increased risk of VOD.
- Other risk factors for VOD in patients treated with BESPONSA included ongoing or prior liver disease, prior HSCT, increased age, later salvage lines, and a greater number of BESPONSA treatment cycles.
- Elevation of liver tests may require dosing interruption, dose reduction, or permanent discontinuation of BESPONSA. Permanently discontinue treatment if VOD occurs. If severe VOD occurs, treat according to standard medical practice [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
INCREASED RISK OF POST-HSCT NON-RELAPSE MORTALITY
- There was higher post-HSCT non-relapse mortality rate in patients receiving BESPONSA, resulting in a higher Day 100 post-HSCT mortality rate [see Warnings and Precautions (5.2)].
- 1. INDICATIONS AND USAGE
-
2. DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
- Pre-medicate before each dose [see Dosage and Administration (2.2)].
- For the first cycle, the recommended total dose of BESPONSA for all patients is 1.8 mg/m2 per cycle, administered as 3 divided doses on Day 1 (0.8 mg/m2), Day 8 (0.5 mg/m2), and Day 15 (0.5 mg/m2). Cycle 1 is 3 weeks in duration, but may be extended to 4 weeks if the patient achieves a complete remission (CR) or complete remission with incomplete hematologic recovery (CRi), and/or to allow recovery from toxicity.
- For subsequent cycles:
- In patients who achieve a CR or CRi, the recommended total dose of BESPONSA is 1.5 mg/m2 per cycle, administered as 3 divided doses on Day 1 (0.5 mg/m2), Day 8 (0.5 mg/m2), and Day 15 (0.5 mg/m2). Subsequent cycles are 4 weeks in duration.
OR - In patients who do not achieve a CR or CRi, the recommended total dose of BESPONSA is 1.8 mg/m2 per cycle given as 3 divided doses on Day 1 (0.8 mg/m2), Day 8 (0.5 mg/m2), and Day 15 (0.5 mg/m2). Subsequent cycles are 4 weeks in duration. Patients who do not achieve a CR or CRi within 3 cycles should discontinue treatment.
- In patients who achieve a CR or CRi, the recommended total dose of BESPONSA is 1.5 mg/m2 per cycle, administered as 3 divided doses on Day 1 (0.5 mg/m2), Day 8 (0.5 mg/m2), and Day 15 (0.5 mg/m2). Subsequent cycles are 4 weeks in duration.
- For patients proceeding to hematopoietic stem cell transplant (HSCT), the recommended duration of treatment with BESPONSA is 2 cycles. A third cycle may be considered for those patients who do not achieve CR or CRi and minimal residual disease (MRD) negativity after 2 cycles [see Warnings and Precautions (5.1)].
- For patients not proceeding to HSCT, additional cycles of treatment, up to a maximum of 6 cycles, may be administered.
Table 1 shows the recommended dosing regimens.
Table 1. Dosing Regimen for Cycle 1 and Subsequent Cycles Depending on Response to Treatment Day 1 Day 8* Day 15* Abbreviations: CR=complete remission; CRi=complete remission with incomplete hematologic recovery. - * +/- 2 days (maintain minimum of 6 days between doses).
- † Dose is based on the patient's body surface area (m2).
- ‡ For patients who achieve a CR or a CRi, and/or to allow for recovery from toxicity, the cycle length may be extended up to 28 days (i.e., 7-day treatment-free interval starting on Day 21).
- § CR is defined as < 5% blasts in the bone marrow and the absence of peripheral blood leukemic blasts, full recovery of peripheral blood counts (platelets ≥ 100 × 109/L and absolute neutrophil counts [ANC] ≥ 1 × 109/L) and resolution of any extramedullary disease.
- ¶ CRi is defined as < 5% blasts in the bone marrow and the absence of peripheral blood leukemic blasts, incomplete recovery of peripheral blood counts (platelets < 100 × 109/L and/or ANC < 1 × 109/L) and resolution of any extramedullary disease.
- # 7-day treatment-free interval starting on Day 21.
Dosing regimen for Cycle 1 All patients: Dose† 0.8 mg/m2 0.5 mg/m2 0.5 mg/m2 Cycle length 21 days‡ Dosing regimen for subsequent cycles depending on response to treatment Patients who have achieved a CR§ or CRi¶: Dose† 0.5 mg/m2 0.5 mg/m2 0.5 mg/m2 Cycle length 28 days# Patients who have not achieved a CR§ or CRi¶: Dose† 0.8 mg/m2 0.5 mg/m2 0.5 mg/m2 Cycle length 28 days# 2.2 Recommended Pre-medications and Cytoreduction
- Premedication with a corticosteroid, antipyretic, and antihistamine is recommended prior to dosing. Patients should be observed during and for at least 1 hour after the end of infusion for symptoms of infusion related reactions [see Warnings and Precautions (5.4)].
- For patients with circulating lymphoblasts, cytoreduction with a combination of hydroxyurea, steroids, and/or vincristine to a peripheral blast count of less than or equal to 10,000/mm3 is recommended prior to the first dose.
2.3 Dose Modification
Modify the dose of BESPONSA for toxicities (see Tables 2–4). BESPONSA doses within a treatment cycle (i.e., Days 8 and/or 15) do not need to be interrupted due to neutropenia or thrombocytopenia, but dosing interruptions within a cycle are recommended for non-hematologic toxicities. If the dose is reduced due to BESPONSA-related toxicity, the dose must not be re-escalated.
Table 2. BESPONSA Dose Modifications for Hematologic Toxicities Criteria BESPONSA Dose Modification(s) Abbreviation: ANC=absolute neutrophil count. - * Platelet count used for dosing should be independent of blood transfusion.
If prior to BESPONSA treatment ANC was greater than or equal to 1 × 109/L If ANC decreases, then interrupt the next cycle of treatment until recovery of ANC to greater than or equal to 1 × 109/L. Discontinue BESPONSA if low ANC persists for greater than 28 days and is suspected to be related to BESPONSA. If prior to BESPONSA treatment platelet count was greater than or equal to 50 × 109/L* If platelet count decreases, then interrupt the next cycle of treatment until platelet count recovers to greater than or equal to 50 × 109/L*. Discontinue BESPONSA if low platelet count persists for greater than 28 days and is suspected to be related to BESPONSA. If prior to BESPONSA treatment ANC was less than 1 × 109/L and/or platelet count was less than 50 × 109/L* If ANC or platelet count decreases, then interrupt the next cycle of treatment until at least one of the following occurs: - - ANC and platelet counts recover to at least baseline levels for the prior cycle, or
- - ANC recovers to greater than or equal to 1 × 109/L and platelet count recovers to greater than or equal to 50 × 109/L*, or
- - Stable or improved disease (based on most recent bone marrow assessment) and the ANC and platelet count decrease is considered to be due to the underlying disease (not considered to be BESPONSA-related toxicity).
Table 3. BESPONSA Dose Modifications for Non-hematologic Toxicities Non-hematologic Toxicity Dose Modification(s) Abbreviations: ALT=alanine aminotransferase; AST=aspartate aminotransferase; ULN=upper limit of normal; VOD=venoocclusive disease. - * Severity grade according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0.
VOD or other severe liver toxicity Permanently discontinue treatment [see Warnings and Precautions (5.1)]. Total bilirubin greater than 1.5 × ULN and AST/ALT greater than 2.5 × ULN Interrupt dosing until recovery of total bilirubin to less than or equal to 1.5 × ULN and AST/ALT to less than or equal to 2.5 × ULN prior to each dose unless due to Gilbert's syndrome or hemolysis. Permanently discontinue treatment if total bilirubin does not recover to less than or equal to 1.5 × ULN or AST/ALT does not recover to less than or equal to 2.5 × ULN [see Warnings and Precautions (5.1)]. Infusion related reaction Interrupt the infusion and institute appropriate medical management. Depending on the severity of the infusion related reaction, consider discontinuation of the infusion or administration of steroids and antihistamines. For severe or life-threatening infusion reactions, permanently discontinue treatment [see Warnings and Precautions (5.4)]. Non-hematologic toxicity greater than or equal to Grade 2* Interrupt treatment until recovery to Grade 1 or pre-treatment grade levels prior to each dose. Table 4. BESPONSA Dose Modifications Depending on Duration of Dosing Interruption Due to Non-Hematologic Toxicity Toxicities Duration of Dose Interruption Due to Toxicity Dose Modification(s) Less than 7 days (within a cycle) Interrupt the next dose (maintain a minimum of 6 days between doses). Greater than or equal to 7 days Omit the next dose within the cycle. Greater than or equal to 14 days Once adequate recovery is achieved, decrease the total dose by 25% for the subsequent cycle. If further dose modification is required, then reduce the number of doses to 2 per cycle for subsequent cycles. If a 25% decrease in the total dose followed by a decrease to 2 doses per cycle is not tolerated, then permanently discontinue treatment. Greater than 28 days Consider permanent discontinuation of treatment. 2.4 Instructions for Reconstitution, Dilution, and Administration
Protect the reconstituted and diluted BESPONSA solutions from light. Do not freeze the reconstituted or diluted solution.
The maximum time from reconstitution through the end of administration should be less than or equal to 8 hours, with less than or equal to 4 hours between reconstitution and dilution.
Reconstitution:
- BESPONSA is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
- Calculate the dose (mg) and number of vials of BESPONSA required.
- Reconstitute each vial with 4 mL of Sterile Water for Injection, USP, to obtain a concentration of 0.25 mg/mL of BESPONSA that delivers 3.6 mL (0.9 mg).
- Gently swirl the vial to aid dissolution. DO NOT SHAKE.
- Inspect the reconstituted solution for particulates and discoloration. The reconstituted solution should be clear to opalescent, colorless to slightly yellow, and essentially free of visible foreign matter.
- See Table 5 for storage times and conditions for the reconstituted solution.
Dilution:
- Calculate the required volume of the reconstituted solution needed to obtain the appropriate dose according to the patient's body surface area. Withdraw this amount from the vial(s) using a syringe. Discard any unused reconstituted BESPONSA solution left in the vial.
- Add reconstituted solution to an infusion container with 0.9% Sodium Chloride Injection, USP, to a make a total volume of 50 mL. An infusion container made of polyvinyl chloride (PVC) (di(2-ethylhexyl)phthalate [DEHP]- or non-DEHP-containing), polyolefin (polypropylene and/or polyethylene), or ethylene vinyl acetate (EVA) is recommended.
- Gently invert the infusion container to mix the diluted solution. DO NOT SHAKE.
- See Table 5 for storage times and conditions for the diluted solution.
Administration:
- See Table 5 for storage times and conditions for prior to and during administration of the diluted solution.
- Filtration of the diluted solution is not required. However, if the diluted solution is filtered, polyethersulfone (PES)-, polyvinylidene fluoride (PVDF)-,or hydrophilic polysulfone (HPS)-based filters are recommended. Do not use filters made of nylon or mixed cellulose ester (MCE).
- Infuse the diluted solution for 1 hour at a rate of 50 mL/h at room temperature (20–25°C; 68–77°F). Infusion lines made of PVC (DEHP- or non-DEHP-containing), polyolefin (polypropylene and/or polyethylene), or polybutadiene are recommended.
Do not mix BESPONSA or administer as an infusion with other medicinal products.
Table 5 shows the storage times and conditions for reconstitution, dilution, and administration of BESPONSA.
Table 5. Storage Times and Conditions for Reconstituted and Diluted BESPONSA Solution Maximum time from reconstitution through end of administration less than or equal to 8 hours* Diluted Solution Reconstituted Solution After Start of Dilution Administration - * With less than or equal to 4 hours between reconstitution and dilution.
BESPONSA contains no bacteriostatic preservatives. Use reconstituted solution immediately or after being refrigerated (2–8°C; 36–46°F) for up to 4 hours. PROTECT FROM LIGHT. DO NOT FREEZE. Use diluted solution immediately or after storage at room temperature (20–25°C; 68–77°F) for up to 4 hours or being refrigerated (2–8°C; 36–46°F) for up to 3 hours. PROTECT FROM LIGHT. DO NOT FREEZE. If the diluted solution is refrigerated (2–8°C; 36–46°F), allow it to equilibrate at room temperature (20–25°C; 68–77°F) for approximately 1 hour prior to administration. Administer diluted solution within 8 hours of reconstitution as a 1-hour infusion at a rate of 50 mL/h at room temperature (20–25°C; 68–77°F). PROTECT FROM LIGHT. - 3. DOSAGE FORMS AND STRENGTHS
- 4. CONTRAINDICATIONS
-
5. WARNINGS AND PRECAUTIONS
5.1 Hepatotoxicity, Including Hepatic Veno-occlusive Disease (VOD) (also known as Sinusoidal Obstruction Syndrome)
In the INO-VATE ALL trial, hepatotoxicity, including severe, life-threatening, and sometimes fatal hepatic VOD was observed in 23/164 patients (14%) in the BESPONSA arm during or following treatment or following a HSCT after completion of treatment. VOD was reported up to 56 days after the last dose during treatment or during follow-up without an intervening HSCT. The median time from subsequent HSCT to onset of VOD was 15 days (range: 3–57 days). In the BESPONSA arm, among the 79 patients who proceeded to a subsequent HSCT, VOD was reported in 18/79 patients (23%), and among all 164 patients treated, VOD was reported in 5/164 patients (3%) during study therapy or in follow-up without an intervening HSCT.
The risk of VOD was greater in patients who underwent HSCT after BESPONSA treatment; use of HSCT conditioning regimens containing 2 alkylating agents (e.g., busulfan in combination with other alkylating agents) and last total bilirubin level greater than or equal to the ULN before HSCT are significantly associated with an increased risk of VOD. Other risk factors for VOD in patients treated with BESPONSA included ongoing or prior liver disease, prior HSCT, increased age, later salvage lines, and a greater number of BESPONSA treatment cycles. Patients who have experienced prior VOD or have serious ongoing hepatic liver disease (e.g., cirrhosis, nodular regenerative hyperplasia, active hepatitis) are at an increased risk for worsening of liver disease, including developing VOD, following treatment with BESPONSA.
Monitor closely for signs and symptoms of VOD; these may include elevations in total bilirubin, hepatomegaly (which may be painful), rapid weight gain, and ascites. Due to the risk of VOD, for patients proceeding to HSCT, the recommended duration of treatment with BESPONSA is 2 cycles; a third cycle may be considered for those patients who do not achieve a CR or CRi and MRD negativity after 2 cycles [see Dosage and Administration (2.1)]. For patients who proceed to HSCT, monitor liver tests closely during the first month post-HSCT, then less frequently thereafter, according to standard medical practice.
In the INO-VATE ALL trial, increases in liver tests were reported. Grade 3/4 AST, ALT, and total bilirubin abnormal liver tests occurred in 7/160 (4%), 7/161 (4%), and 8/161 patients (5%), respectively.
In all patients, monitor liver tests, including ALT, AST, total bilirubin, and alkaline phosphatase, prior to and following each dose of BESPONSA. Elevations of liver tests may require dosing interruption, dose reduction, or permanent discontinuation of BESPONSA [see Dosage and Administration (2.3)].
5.2 Increased Risk of Post-Transplant Non-Relapse Mortality
In the INO-VATE ALL trial, a higher post-HSCT non-relapse mortality rate was observed in patients receiving BESPONSA compared to the Investigator's choice of chemotherapy arm, resulting in a higher Day 100 post-HSCT mortality rate.
Overall, 79/164 patients (48%) in the BESPONSA arm and 35/162 patients (22%) in the Investigator's choice of chemotherapy arm had a follow-up HSCT. The post-HSCT non-relapse mortality rate was 31/79 (39%) and 8/35 (23%) in the BESPONSA arm compared to the Investigator's choice of chemotherapy arm, respectively.
In the BESPONSA arm, the most common causes of post-HSCT non-relapse mortality included VOD and infections. Five of the 18 VOD events that occurred post-HSCT were fatal. In the BESPONSA arm, among patients with ongoing VOD at time of death, 6 patients died due to multiorgan failure (MOF) or infection (3 patients died due to MOF, 2 patients died due to infection, and 1 patient died due to MOF and infection).
Monitor closely for toxicities post-HSCT, including signs and symptoms of infection and VOD [see Warnings and Precautions (5.1, 5.3)].
5.3 Myelosuppression
In the INO-VATE ALL trial, myelosuppression was observed in patients receiving BESPONSA [see Adverse Reactions (6.1)].
Thrombocytopenia and neutropenia were reported in 83/164 patients (51%) and 81/164 patients (49%), respectively. Grade 3 thrombocytopenia and neutropenia were reported in 23/164 patients (14%) and 33/164 patients (20%), respectively. Grade 4 thrombocytopenia and neutropenia were reported in 46/164 patients (28%) and 45/164 patients (27%), respectively. Febrile neutropenia, which may be life-threatening, was reported in 43/164 patients (26%). For patients who were in CR or CRi at the end of treatment, the recovery of platelet counts to > 50,000/mm3 was later than 45 days after the last dose in 15/164 patients (9%) who received BESPONSA and 3/162 patients (2%) who received Investigator's choice of chemotherapy.
Complications associated with myelosuppression (including infections and bleeding/hemorrhagic events) were observed in patients receiving BESPONSA [see Adverse Reactions (6.1)]. Infections, including serious infections, some of which were life-threatening or fatal, were reported in 79/164 patients (48%). Fatal infections, including pneumonia, neutropenic sepsis, sepsis, septic shock, and pseudomonal sepsis, were reported in 8/164 patients (5%). Bacterial, viral, and fungal infections were reported.
Hemorrhagic events were reported in 54/164 patients (33%). Grade 3 or 4 hemorrhagic events were reported in 8/164 patients (5%). One Grade 5 (fatal) hemorrhagic event (intra-abdominal hemorrhage) was reported in 1/164 patients (1%). The most common hemorrhagic event was epistaxis which was reported in 24/164 patients (15%).
Monitor complete blood counts prior to each dose of BESPONSA and monitor for signs and symptoms of infection, bleeding/hemorrhage, or other effects of myelosuppression during treatment with BESPONSA. As appropriate, administer prophylactic anti-infectives and employ surveillance testing during and after treatment with BESPONSA. Management of severe infection, bleeding/hemorrhage, or other effects of myelosuppression, including severe neutropenia or thrombocytopenia, may require dosing interruption, dose reduction, or permanent discontinuation of BESPONSA [see Dosage and Administration (2.3)].
5.4 Infusion Related Reactions
In the INO-VATE ALL trial, infusion related reactions were observed in patients who received BESPONSA. Infusion related reactions (all Grade 2) were reported in 4/164 patients (2%). Infusion related reactions generally occurred in Cycle 1 shortly after the end of the BESPONSA infusion and resolved spontaneously or with medical management.
Premedicate with a corticosteroid, antipyretic, and antihistamine prior to dosing [see Dosage and Administration (2.2)].
Monitor patients closely during and for at least 1 hour after the end of infusion for the potential onset of infusion related reactions, including symptoms such as fever, chills, rash, or breathing problems. Interrupt infusion and institute appropriate medical management if an infusion related reaction occurs. Depending on the severity of the infusion related reaction, consider discontinuation of the infusion or administration of steroids and antihistamines. For severe or life-threatening infusion reactions, permanently discontinue BESPONSA [see Dosage and Administration (2.3)].
5.5 QT Interval Prolongation
In the INO-VATE ALL trial, increases in QT interval corrected for heart rate using Fridericia's formula (QTcF) of ≥ to 60 msec from baseline were measured in 4/162 patients (3%). No patients had QTcF values greater than 500 msec [see Clinical Pharmacology (12.2)]. Grade 2 QT prolongation was reported in 2/164 patients (1%). No ≥ Grade 3 QT prolongation or events of Torsade de Pointes were reported [see Adverse Reactions (6.1)].
Administer BESPONSA with caution in patients who have a history of or predisposition for QTc prolongation, who are taking medicinal products that are known to prolong QT interval [see Drug Interactions (7)], and in patients with electrolyte disturbances [see Drug Interactions (7)]. Obtain electrocardiograms (ECGs) and electrolytes prior to the start of treatment, after initiation of any drug known to prolong QTc, and periodically monitor as clinically indicated during treatment [see Drug Interactions (7), Clinical Pharmacology (12.2)]).
5.6 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, BESPONSA can cause embryo-fetal harm when administered to a pregnant woman. In animal studies, inotuzumab ozogamicin caused embryo-fetal toxicities, starting at a dose that was approximately 0.4 times the exposure in patients at the maximum recommended dose, based on the area under the concentration-time curve (AUC). Advise females of reproductive potential to use effective contraception during treatment with BESPONSA and for at least 8 months after the final dose of BESPONSA. Advise males with female partners of reproductive potential to use effective contraception during treatment with BESPONSA and for at least 5 months after the last dose of BESPONSA. Apprise pregnant women of the potential risk to the fetus. Advise women to contact their healthcare provider if they become pregnant or if pregnancy is suspected during treatment with BESPONSA [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1), Nonclinical Toxicology (13.1)].
-
6. ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Hepatotoxicity, including hepatic VOD (also known as SOS) [see Warnings and Precautions (5.1)]
- Increased risk of post-transplant non-relapse mortality [see Warnings and Precautions (5.2)]
- Myelosuppression [see Warnings and Precautions (5.3)]
- Infusion related reactions [see Warnings and Precautions (5.4)]
- QT interval prolongation [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse reactions described in this section reflect exposure to BESPONSA in 164 patients with relapsed or refractory ALL who participated in a randomized clinical study of BESPONSA versus Investigator's choice of chemotherapy (fludarabine + cytarabine + granulocyte colony-stimulating factor [FLAG], mitoxantrone + cytarabine [MXN/Ara-C], or high dose cytarabine [HIDAC]) (INO-VATE ALL Trial [NCT01564784]) [see Clinical Studies (14)].
Of the 164 patients who received BESPONSA, the median age was 47 years (range: 18–78 years), 56% were male, 68% had received 1 prior treatment regimen for ALL, 31% had received 2 prior treatment regimens for ALL, 68% were White, 19% were Asian, and 2% were Black.
In patients who received BESPONSA, the median duration of treatment was 8.9 weeks (range: 0.1–26.4 weeks), with a median of 3 treatment cycles started in each patient. In patients who received Investigator's choice of chemotherapy, the median duration of treatment was 0.9 weeks (range: 0.1–15.6 weeks), with a median of 1 treatment cycle started in each patient.
In patients who received BESPONSA, the most common (≥ 20%) adverse reactions were thrombocytopenia, neutropenia, infection, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, gamma-glutamyltransferase increased, and hyperbilirubinemia.
In patients who received BESPONSA, the most common (≥ 2%) serious adverse reactions were infection, febrile neutropenia, hemorrhage, abdominal pain, pyrexia, VOD, and fatigue.
In patients who received BESPONSA, the most common (≥ 2%) adverse reactions reported as the reason for permanent discontinuation were infection (6%), thrombocytopenia (2%), hyperbilirubinemia (2%), transaminases increased (2%), and hemorrhage (2%); the most common (≥ 5%) adverse reactions reported as the reason for dosing interruption were neutropenia (17%), infection (10%), thrombocytopenia (10%), transaminases increased (6%), and febrile neutropenia (5%); and the most common (≥ 1%) adverse reactions reported as the reason for dose reduction were neutropenia (1%), thrombocytopenia (1%), and transaminases increased (1%).
VOD was reported in 23/164 patients (14%) who received BESPONSA during or following treatment or following a HSCT after completion of treatment [see Warnings and Precautions (5.1)].
Table 6 shows the adverse reactions with ≥ 10% incidence reported in patients with relapsed or refractory ALL who received BESPONSA or Investigator's choice of chemotherapy.
Table 6. Adverse Reactions With ≥ 10% Incidence* in Patients With Relapsed or Refractory B-Cell Precursor ALL Who Received BESPONSA or Investigator's Choice of Chemotherapy (FLAG, MXN/Ara-C, or HIDAC) Body System
Adverse ReactionBESPONSA
(N=164)FLAG, MXN/Ara-C, or HIDAC
(N=143†)All Grades ≥ Grade 3 All Grades ≥ Grade 3 % % % % Adverse reactions included treatment-emergent all-causality events that commenced on or after Cycle 1 Day 1 within 42 days after the last dose of BESPONSA, but prior to the start of a new anticancer treatment (including HSCT).
Preferred terms were retrieved by applying the Medical Dictionary for Regulatory Activities (MedDRA) version 18.1.
Severity grade of adverse reactions were according to NCI CTCAE version 3.0.
Abbreviations: ALL=acute lymphoblastic leukemia; FLAG=fludarabine + cytarabine + granulocyte colony-stimulating factor; HIDAC=high dose cytarabine; HSCT=hematopoietic stem cell transplant; MXN/Ara-C=mitoxantrone + cytarabine; N=number of patients; NCI CTCAE=National Cancer Institute Common Toxicity Criteria for Adverse Events.- * Only adverse reactions with ≥ 10% incidence in the BESPONSA arm are included.
- † 19 patients randomized to FLAG, MXN/Ara-C, or HIDAC did not receive treatment.
- ‡ Infection includes any reported preferred terms for BESPONSA retrieved in the System Organ Class Infections and infestations.
- § Thrombocytopenia includes the following reported preferred terms: Platelet count decreased and Thrombocytopenia.
- ¶ Neutropenia includes the following reported preferred terms: Neutropenia and Neutrophil count decreased.
- # Anemia includes the following reported preferred terms: Anemia and Hemoglobin decreased.
- Þ Leukopenia includes the following reported preferred terms: Leukopenia, Monocytopenia, and White blood cell count decreased.
- ß Lymphopenia includes the following reported preferred terms: B-lymphocyte count decreased, Lymphocyte count decreased, and Lymphopenia.
- à Headache includes the following reported preferred terms: Headache, Migraine, and Sinus headache.
- è Hemorrhage includes reported preferred terms for BESPONSA retrieved in the Standard MedDRA Query (narrow) for Hemorrhage terms (excluding laboratory terms), resulting in the following preferred terms: Conjunctival hemorrhage, Contusion, Ecchymosis, Epistaxis, Eyelid bleeding, Gastrointestinal hemorrhage, Gastritis hemorrhagic, Gingival bleeding, Hematemesis, Hematochezia, Hematotympanum, Hematuria, Hemorrhage intracranial, Hemorrhage subcutaneous, Hemorrhoidal hemorrhage, Intra-abdominal hemorrhage, Lip hemorrhage, Lower gastrointestinal hemorrhage, Mesenteric hemorrhage, Metrorrhagia, Mouth hemorrhage, Muscle hemorrhage, Oral mucosa hematoma, Petechiae, Post-procedural hematoma, Rectal hemorrhage, Shock hemorrhagic, Subcutaneous hematoma, Subdural hematoma, Upper gastrointestinal hemorrhage, and Vaginal hemorrhage.
- ð Abdominal pain includes the following reported preferred terms: Abdominal pain, Abdominal pain lower, Abdominal pain upper, Abdominal tenderness, Esophageal pain, and Hepatic pain.
- ø Stomatitis includes the following reported preferred terms: Aphthous ulcer, Mucosal inflammation, Mouth ulceration, Oral pain, Oropharyngeal pain, and Stomatitis.
- ý Fatigue includes the following reported preferred terms: Asthenia and Fatigue.
- £ Transaminases increased includes the following reported preferred terms: Aspartate aminotransferase increased, Alanine aminotransferase increased, Hepatocellular injury, and Hypertransaminasemia.
Infections Infection‡ 48 28 76 54 Blood and lymphatic system disorders Thrombocytopenia§ 51 42 61 59 Neutropenia¶ 49 48 45 43 Anemia# 36 24 59 47 LeukopeniaÞ 35 33 43 42 Febrile neutropenia 26 26 53 53 Lymphopeniaß 18 16 27 26 Metabolism and nutrition disorders Decreased appetite 12 1 13 2 Nervous system disorders Headacheà 28 2 27 1 Vascular disorders Hemorrhageè 33 5 28 5 Gastrointestinal disorders Nausea 31 2 46 0 Abdominal painð 23 3 23 1 Diarrhea 17 1 38 1 Constipation 16 0 24 0 Vomiting 15 1 24 0 Stomatitisø 13 2 26 3 Hepatobiliary disorders Hyperbilirubinemia 21 5 17 6 General disorders and administration site conditions Fatigueý 35 5 25 3 Pyrexia 32 3 42 6 Chills 11 0 11 0 Investigations Transaminases increased£ 26 7 13 5 Gamma-glutamyltransferase increased 21 10 8 4 Alkaline phosphatase increased 13 2 7 0 Additional adverse reactions (all grades) that were reported in less than 10% of patients treated with BESPONSA included: lipase increased (9%), abdominal distension (6%), amylase increased (5%), hyperuricemia (4%), ascites (4%), infusion related reaction (2%; includes the following: hypersensitivity and infusion related reaction), pancytopenia (2%; includes the following: bone marrow failure, febrile bone marrow aplasia, and pancytopenia), tumor lysis syndrome (2%), and electrocardiogram QT prolonged (1%).
Table 7 shows the clinically important laboratory abnormalities reported in patients with relapsed or refractory ALL who received BESPONSA or Investigator's choice of chemotherapy.
Table 7. Laboratory Abnormalities in Patients With Relapsed or Refractory B-Cell Precursor ALL Who Received BESPONSA or Investigator's Choice of Chemotherapy (FLAG, MXN/Ara-C, or HIDAC) BESPONSA FLAG, MXN/Ara-C, or HIDAC All Grades Grade 3/4 All Grades Grade 3/4 Laboratory Abnormality* N % % N % % Severity grade of laboratory abnormalities according to NCI CTCAE version 3.0.
Abbreviations: ALL=acute lymphoblastic leukemia; ALP=alkaline phosphatase; ALT=alanine aminotransferase; AST=aspartate aminotransferase; FLAG=fludarabine + cytarabine + granulocyte colony-stimulating factor; GGT=gamma-glutamyltransferase; HIDAC=high dose cytarabine; MXN/Ara-C=mitoxantrone + cytarabine; N=number of patients; NCI CTCAE=National Cancer Institute Common Toxicity Criteria for Adverse Events.- * Laboratory abnormalities were summarized up to the end of treatment + 42 days but prior to the start of a new anti-cancer therapy.
Hematology Platelet count decreased 161 98 76 142 100 99 Hemoglobin decreased 161 94 40 142 100 70 Leukocytes decreased 161 95 82 142 99 98 Neutrophil count decreased 160 94 86 130 93 88 Lymphocytes (absolute) decreased 160 93 71 127 97 91 Chemistry GGT increased 148 67 18 111 68 17 AST increased 160 71 4 134 38 4 ALP increased 158 57 1 133 52 3 ALT increased 161 49 4 137 46 4 Blood bilirubin increased 161 36 5 138 35 6 Lipase increased 139 32 13 90 20 2 Hyperuricemia 158 16 3 122 11 0 Amylase increased 143 15 2 102 9 1 6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to inotuzumab ozogamicin in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
In clinical studies of BESPONSA in patients with relapsed or refractory ALL, the immunogenicity of BESPONSA was evaluated using an electrochemiluminescence (ECL)-based immunoassay to test for anti-inotuzumab ozogamicin antibodies. For patients whose sera tested positive for anti-inotuzumab ozogamicin antibodies, a cell-based luminescence assay was performed to detect neutralizing antibodies.
In clinical studies of BESPONSA in patients with relapsed or refractory ALL, 7/236 patients (3%) tested positive for anti-inotuzumab ozogamicin antibodies. No patients tested positive for neutralizing anti-inotuzumab ozogamicin antibodies. In patients who tested positive for anti-inotuzumab ozogamicin antibodies, the presence of anti-inotuzumab ozogamicin antibodies did not affect clearance following BESPONSA treatment.
-
7. DRUG INTERACTIONS
Drugs That Prolong the QT Interval
Concomitant use of BESPONSA with drugs known to prolong the QT interval or induce Torsades de Pointes may increase the risk of a clinically significant QTc interval prolongation [see Clinical Pharmacology (12.2)]. Discontinue or use alternative concomitant drugs that do not prolong QT/QTc interval while the patient is using BESPONSA. When it is not feasible to avoid concomitant use of drugs known to prolong QT/QTc, obtain ECGs and electrolytes prior to the start of treatment, after initiation of any drug known to prolong QTc, and periodically monitor as clinically indicated during treatment [see Warnings and Precautions (5.5)].
-
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action and findings from animal studies [see Clinical Pharmacology (12.1), Nonclinical Toxicology (13.1)], BESPONSA can cause embryo-fetal harm when administered to a pregnant woman. There are no available data on BESPONSA use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. In rat embryo-fetal development studies, inotuzumab ozogamicin caused embryo-fetal toxicity at maternal systemic exposures that were ≥ 0.4 times the exposure in patients at the maximum recommended dose, based on AUC [see Data]. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, advise the patient of the potential risk to a fetus.
Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2–4% and 15–20%, respectively.
Data
Animal Data
In embryo-fetal development studies in rats, pregnant animals received daily intravenous doses of inotuzumab ozogamicin up to 0.36 mg/m2 during the period of organogenesis. Embryo-fetal toxicities including increased resorptions and fetal growth retardation as evidenced by decreased live fetal weights and delayed skeletal ossification were observed at ≥ 0.11 mg/m2 (approximately 2 times the exposure in patients at the maximum recommended dose, based on AUC). Fetal growth retardation also occurred at 0.04 mg/m2 (approximately 0.4 times the exposure in patients at the maximum recommended dose, based on AUC).
In an embryo-fetal development study in rabbits, pregnant animals received daily intravenous doses up to 0.15 mg/m2 (approximately 3 times the exposure in patients at the maximum recommended dose, based on AUC) during the period of organogenesis. At a dose of 0.15 mg/m2, slight maternal toxicity was observed in the absence of any effects on embryo-fetal development.
8.2 Lactation
Risk Summary
There are no data on the presence of inotuzumab ozogamicin or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for adverse reactions in breastfed infants, advise women not to breastfeed during treatment with BESPONSA and for at least 2 months after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Based on its mechanism of action and findings from animal studies, BESPONSA can cause embryo-fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1), Nonclinical Toxicology (13.1)]. Verify the pregnancy status of females of reproductive potential prior to initiating BESPONSA.
Contraception
Females
Advise females of reproductive potential to avoid becoming pregnant while receiving BESPONSA. Advise females of reproductive potential to use effective contraception during treatment with BESPONSA and for at least 8 months after the last dose [see Nonclinical Toxicology (13.1)].
Males
Advise males with female partners of reproductive potential to use effective contraception during treatment with BESPONSA and for at least 5 months after the last dose [see Nonclinical Toxicology (13.1)].
Infertility
Females
Based on findings in animals, BESPONSA may impair fertility in females of reproductive potential [see Nonclinical Toxicology (13.1)].
Males
Based on findings in animals, BESPONSA may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.5 Geriatric Use
In the INO-VATE ALL trial, 30/164 patients (18%) treated with BESPONSA were ≥ 65 years of age. No differences in responses were identified between older and younger patients.
Based on a population pharmacokinetic analysis in 765 patients, no adjustment to the starting dose is required based on age [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
Based on a population pharmacokinetic analysis, the clearance of inotuzumab ozogamicin in patients with mild hepatic impairment (total bilirubin less than or equal to ULN and AST greater than ULN, or total bilirubin greater than 1.0–1.5 × ULN and AST any level; n=150) was similar to patients with normal hepatic function (total bilirubin/AST less than or equal to ULN; n=611). In patients with moderate (total bilirubin greater than 1.5–3 × ULN and AST any level; n=3) and severe hepatic impairment (total bilirubin greater than 3 × ULN and AST any level; n=1), inotuzumab ozogamicin clearance did not appear to be reduced [see Clinical Pharmacology (12.3)].
No adjustment to the starting dose is required when administering BESPONSA to patients with total bilirubin less than or equal to 1.5 × ULN and AST/ALT less than or equal to 2.5 × ULN [see Dosage and Administration (2.3)]. There is limited safety information available in patients with total bilirubin greater than 1.5 × ULN and/or AST/ALT greater than 2.5 × ULN prior to dosing. Interrupt dosing until recovery of total bilirubin to less than or equal to 1.5 × ULN and AST/ALT to less than or equal to 2.5 × ULN prior to each dose unless due to Gilbert's syndrome or hemolysis. Permanently discontinue treatment if total bilirubin does not recover to less than or equal to 1.5 × ULN or AST/ALT does not recover to less than or equal to 2.5 × ULN [see Dosage and Administration (2.3), Warnings and Precautions (5.1)].
-
11. DESCRIPTION
Inotuzumab ozogamicin is a CD22-directed antibody-drug conjugate (ADC) consisting of 3 components: 1) the recombinant humanized immunoglobulin class G subtype 4 (IgG4) kappa antibody inotuzumab, specific for human CD22, 2) N-acetyl-gamma-calicheamicin that causes double-stranded DNA breaks, and 3) an acid-cleavable linker composed of the condensation product of 4-(4'-acetylphenoxy)-butanoic acid (AcBut) and 3-methyl-3-mercaptobutane hydrazide (known as dimethylhydrazide) that covalently attaches N-acetyl-gamma-calicheamicin to inotuzumab.
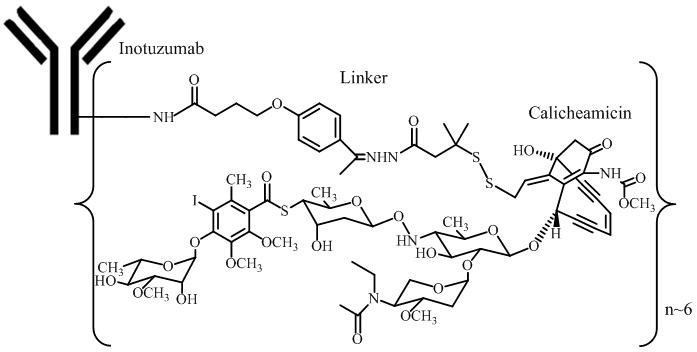
Inotuzumab ozogamicin has an approximate molecular weight of 160 kDa. The average number of calicheamicin derivative molecules conjugated to each inotuzumab molecule is approximately 6 with a distribution from 2–8. Inotuzumab ozogamicin is produced by chemical conjugation of the antibody and small molecule components. The antibody is produced by mammalian (Chinese hamster ovary) cells, and the semisynthetic calicheamicin derivative is produced by microbial fermentation followed by synthetic modification.
BESPONSA (inotuzumab ozogamicin) for Injection is supplied as a sterile, white to off-white, preservative-free, lyophilized powder for intravenous administration. Each single-dose vial delivers 0.9 mg inotuzumab ozogamicin. Inactive ingredients are polysorbate 80 (0.36 mg), sodium chloride (2.16 mg), sucrose (180 mg), and tromethamine (8.64 mg). After reconstitution with 4 mL of Sterile Water for Injection, USP, the final concentration is 0.25 mg/mL of inotuzumab ozogamicin with a deliverable volume of 3.6 mL (0.9 mg) and a pH of approximately 8.0.
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Inotuzumab ozogamicin is a CD22-directed antibody-drug conjugate (ADC). Inotuzumab recognizes human CD22. The small molecule, N-acetyl-gamma-calicheamicin, is a cytotoxic agent that is covalently attached to the antibody via a linker. Nonclinical data suggest that the anticancer activity of inotuzumab ozogamicin is due to the binding of the ADC to CD22-expressing tumor cells, followed by internalization of the ADC-CD22 complex, and the intracellular release of N-acetyl-gamma-calicheamicin dimethylhydrazide via hydrolytic cleavage of the linker. Activation of N-acetyl-gamma-calicheamicin dimethylhydrazide induces double-strand DNA breaks, subsequently inducing cell cycle arrest and apoptotic cell death.
12.2 Pharmacodynamics
During the treatment period, the pharmacodynamic response to BESPONSA was characterized by the depletion of CD22-positive leukemic blasts.
Cardiac Electrophysiology
In a randomized clinical study in patients with relapsed or refractory ALL, increases in QTcF of ≥ 60 msec from baseline were measured in 4/162 patients (3%) in the BESPONSA arm and 3/124 patients (2%) in the Investigator's choice of chemotherapy arm. Increases in QTcF of > 500 msec were observed in none of the patients in the BESPONSA arm and 1/124 patients (1%) in the Investigator's choice of chemotherapy arm. Central tendency analysis of the QTcF interval changes from baseline showed that the highest mean (upper bound of the 2-sided 90% CI) for QTcF was 15.3 (21.1) msec, which was observed at Cycle 4/Day 1/1 hour in the BESPONSA arm [see Warnings and Precautions (5.5)].
12.3 Pharmacokinetics
The mean Cmax of inotuzumab ozogamicin was 308 ng/mL. The mean simulated total AUC per cycle was 100,000 ng∙h/mL. In patients with relapsed or refractory ALL, steady-state drug concentration was achieved by Cycle 4. Following administration of multiple doses, a 5.3 times accumulation of inotuzumab ozogamicin was predicted by Cycle 4.
Distribution
N-acetyl-gamma-calicheamicin dimethylhydrazide is approximately 97% bound to human plasma proteins in vitro. In humans, the total volume of distribution of inotuzumab ozogamicin was approximately 12 L.
Elimination
The pharmacokinetics of inotuzumab ozogamicin was well characterized by a 2-compartment model with linear and time-dependent clearance components. In 234 patients with relapsed or refractory ALL, the clearance of inotuzumab ozogamicin at steady state was 0.0333 L/h and the terminal half-life (t½) was 12.3 days. Following administration of multiple doses, a 5.3 times accumulation of inotuzumab ozogamicin was predicted by Cycle 4.
Metabolism
In vitro, N-acetyl-gamma-calicheamicin dimethylhydrazide was primarily metabolized via nonenzymatic reduction. In humans, N-acetyl-gamma-calicheamicin dimethylhydrazide serum levels were typically below the limit of quantitation.
Specific Populations
The effect of intrinsic factors on inotuzumab ozogamicin pharmacokinetics was assessed using a population pharmacokinetic analysis unless otherwise specified. Age (18 to 92 years of age), sex, and race (Asian versus non-Asian [Caucasian, Black, and Unspecified]) had no clinically significant effect on the pharmacokinetics of inotuzumab ozogamicin. Body surface area was found to significantly affect inotuzumab ozogamicin disposition. BESPONSA is dosed based on body surface area [see Dosage and Administration (2.1)].
Patients with Renal Impairment
The clearance of inotuzumab ozogamicin in patients with mild renal impairment (creatinine clearance [CLcr; based on the Cockcroft-Gault formula] 60–89 mL/min; n=237), moderate renal impairment (CLcr 30–59 mL/min; n=122), or severe renal impairment (CLcr 15–29 mL/min; n=4) was similar to patients with normal renal function (CLcr ≥90 mL/min; n=402). The safety and efficacy of inotuzumab ozogamicin in patients with end stage renal disease with or without hemodialysis is unknown.
Patients with Hepatic Impairment
The clearance of inotuzumab ozogamicin in patients with mild hepatic impairment (total bilirubin ≤ULN and AST > ULN, or total bilirubin >1.0–1.5 × ULN and AST any level; n=150) was similar to patients with normal hepatic function (total bilirubin/AST ≤ULN; n=611). There is insufficient data in patients with moderate and severe hepatic impairment (total bilirubin >1.5 ULN).
Drug Interactions
In vitro
Effect of Metabolic Pathways and Transporter Systems on BESPONSA
N-acetyl-gamma-calicheamicin dimethylhydrazide is a substrate of P-glycoprotein (P-gp).
Effect of BESPONSA on Metabolic Pathways and Transporter Systems
At clinically relevant concentrations, N-acetyl-gamma-calicheamicin dimethylhydrazide had a low potential to:
- Inhibit cytochrome P450 (CYP 450) Enzymes: CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5.
- Induce CYP450 Enzymes: CYP1A2, CYP2B6, and CYP3A4.
- Inhibit UGT Enzymes: UGT1A1, UGT1A4, UGT1A6, UGT1A9, and UGT2B7.
- Inhibit Drug Transporters: P-gp, breast cancer resistance protein (BCRP), organic anion transporter (OAT)1 and OAT3, organic cation transporter (OCT)2, and organic anion transporting polypeptide (OATP)1B1 and OATP1B3.
At clinically relevant concentrations, inotuzumab ozogamicin had a low potential to:
- Inhibit CYP450 Enzymes: CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4/5.
- Induce CYP450 Enzymes: CYP1A2, CYP2B6, and CYP3A4.
-
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Formal carcinogenicity studies have not been conducted with inotuzumab ozogamicin. In toxicity studies, rats were dosed weekly for 4 or 26 weeks with inotuzumab ozogamicin at doses up to 4.1 mg/m2 and 0.73 mg/m2, respectively. After 26 weeks of dosing, rats developed hepatocellular adenomas in the liver at 0.73 mg/m2 (approximately 2 times the exposure in patients at the maximum recommended dose, based on AUC).
Inotuzumab ozogamicin was clastogenic in vivo in the bone marrow of male mice that received single doses ≥1.1 mg/m2. This is consistent with the known induction of DNA breaks by calicheamicin. N-acetyl-gamma-calicheamicin dimethylhydrazide (the cytotoxic agent released from inotuzumab ozogamicin) was mutagenic in an in vitro bacterial reverse mutation (Ames) assay.
In a female fertility and early embryonic development study, female rats were administered daily intravenous doses of inotuzumab ozogamicin up to 0.11 mg/m2 for 2 weeks before mating through Day 7 of pregnancy. An increase in the proportion of resorptions and decrease in the number of viable embryos and gravid uterine weights were observed at the 0.11 mg/m2 dose level (approximately 2 times the exposure in patients at the maximum recommended dose, based on AUC). Additional findings in female reproductive organs occurred in repeat-dose toxicology studies and included decreased ovarian and uterine weights, and ovarian and uterine atrophy. Findings in male reproductive organs occurred in repeat-dose toxicology studies and included decreased testicular weights, testicular degeneration, hypospermia, and prostatic and seminal vesicle atrophy. Testicular degeneration and hypospermia were nonreversible following a 4-week nondosing period. In the chronic studies of 26-weeks duration, adverse effects on reproductive organs occurred at ≥0.07 mg/m2 in male rats and at 0.73 mg/m2 in female monkeys [see Use in Specific Populations (8.3)].
-
14. CLINICAL STUDIES
Patients With Relapsed or Refractory ALL – INO-VATE ALL
The safety and efficacy of BESPONSA were evaluated in INO-VATE ALL (NCT01564784) a randomized (1:1), open-label, international, multicenter study in patients with relapsed or refractory ALL. Patients were stratified at randomization based on duration of first remission (< 12 months or ≥ 12 months, salvage treatment (Salvage 1 or 2) and patient age at randomization (< 55 or ≥ 55 years). Eligible patients were ≥ 18 years of age with Philadelphia chromosome-negative or Philadelphia chromosome-positive relapsed or refractory B-cell precursor ALL. All patients were required to have ≥ 5% bone marrow blasts and to have received 1 or 2 previous induction chemotherapy regimens for ALL. Patients with Philadelphia chromosome-positive B-cell precursor ALL were required to have disease that failed treatment with at least 1 tyrosine kinase inhibitor and standard chemotherapy. Table 1 shows the dosing regimen used to treat patients.
Among all 326 patients who were randomized to receive BESPONSA (N=164) or Investigator's choice of chemotherapy (N=162), 215 patients (66%) had received 1 prior treatment regimen for ALL and 108 patients (33%) had received 2 prior treatment regimens for ALL. The median age was 47 years (range: 18–79 years), 276 patients (85%) had Philadelphia chromosome-negative ALL, 206 patients (63%) had a duration of first remission < 12 months, and 55 patients (17%) had undergone a HSCT prior to receiving BESPONSA or Investigator's choice of chemotherapy. The two treatment groups were generally balanced with respect to the baseline demographics and disease characteristics.
All evaluable patients had B-cell precursor ALL that expressed CD22, with ≥ 90% of evaluable patients exhibiting ≥ 70% leukemic blast CD22 positivity prior to treatment, as assessed by flow cytometry performed at a central laboratory.
The efficacy of BESPONSA was established on the basis of CR, the duration of CR, and proportion of MRD-negative CR (< 1 × 10-4 of bone marrow nucleated cells by flow cytometry) in the first 218 patients randomized. CR, duration of remission (DoR), and MRD results in the initial 218 randomized patients were consistent with those seen in all 326 randomized patients.
Among the initial 218 randomized patients, 64/88 (73%) and 21/88 (24%) of responding patients per EAC achieved CR/CRi in Cycles 1 and 2, respectively, in the BESPONSA arm, and 29/32 (91%) and 1/32 (3%) of responding patients per EAC achieved a CR/CRi in Cycles 1 and 2, respectively, in the Investigator's choice of chemotherapy arm.
Table 8 shows the efficacy results from this study.
Table 8. Efficacy Results in Patients With Relapsed or Refractory B-Cell Precursor ALL Who Received BESPONSA or Investigator's Choice of Chemotherapy (FLAG, MXN/Ara-C, or HIDAC) CR* CRi† CR/CRi*,† BESPONSA
(N=109)HIDAC, FLAG, or MXN/Ara-C
(N=109)BESPONSA
(N=109)HIDAC, FLAG or MXN/Ara-C
(N=109)BESPONSA
(N=109)HIDAC, FLAG, or MXN/Ara-C
(N=109)Abbreviations: CI=confidence interval; CR=complete remission; CRi=complete remission with incomplete hematologic recovery; DoR=duration of remission; EAC=Endpoint Adjudication Committee; FLAG=fludarabine + cytarabine + granulocyte colony-stimulating factor; HIDAC=high-dose cytarabine; HR=hazard ratio; MRD=minimal residual disease; MXN/AraC=mitoxantrone + cytarabine; N/n=number of patients; OS=overall survival; PFS=progression-free survival. - * CR, per EAC, was defined as < 5% blasts in the bone marrow and the absence of peripheral blood leukemic blasts, full recovery of peripheral blood counts (platelets ≥ 100 × 109/L and absolute neutrophil counts [ANC] ≥ 1 × 109/L) and resolution of any extramedullary disease.
- † CRi, per EAC, was defined as < 5% blasts in the bone marrow and the absence of peripheral blood leukemic blasts, incomplete recovery of peripheral blood counts (platelets < 100 × 109/L and/or ANC < 1 × 109/L) and resolution of any extramedullary disease.
- ‡ 1-sided p-value using Chi-squared test.
- § DoR, based on a later cutoff date than the CR/CRi, was defined for patients who achieved CR/CRi per Investigator's assessment as time since first response of CR* or CRi† per Investigator's assessment to the date of a PFS event or censoring date if no PFS event was documented.
- ¶ MRD-negativity was defined by flow cytometry as leukemic cells comprising < 1 × 10-4 (< 0.01%) of bone marrow nucleated cells.
- # Rate was defined as the number of patients who achieved MRD negativity divided by the total number of patients who achieved CR/CRi per EAC.
Responding (CR/CRi) patients n (%)
[95% CI]39 (35.8)
[26.8–45.5]19 (17.4)
[10.8–25.9]49 (45.0)
[35.4–54.8]13 (11.9)
[6.5–19.5]88 (80.7)
[72.1–87.7]32 (29.4)
[21.0–38.8]p-value‡ <0.0001 DoR§ n 39 18 45 14 84 32 Median, months
[95% CI]8.0
[4.9–10.4]4.9
[2.9–7.2]4.6
[3.7–5.7]2.9
[0.6–5.7]5.4
[4.2–8.0]3.5
[2.9–6.6]MRD-negativity¶ n 35 6 34 3 69 9 Rate# (%)
[95% CI]35/39 (89.7)
[75.8–97.1]6/19 (31.6)
[12.6–56.6]34/49 (69.4)
[54.6–81.7]3/13 (23.1)
[5.0–53.8]69/88 (78.4)
[68.4–86.5]9/32 (28.1)
[13.7–46.7]Among the initial 218 patients, as per EAC assessment, 32/109 patients (29%) in the BESPONSA arm achieved complete remission with partial hematologic recovery (CRh; defined as <5% blasts in the bone marrow, ANC > 0.5 × 109/L, and platelet counts > 50 × 109/L but not meeting full recovery of peripheral blood counts) versus 6/109 patients (6%) in the Investigator's choice of chemotherapy arm, and 71/109 patients (65%) in the BESPONSA arm achieved CR/CRh versus 25/109 patients (23%) in the Investigator's choice of chemotherapy arm.
Overall, 79/164 patients (48%) in the BESPONSA arm and 35/162 patients (22%) in the Investigator's choice of chemotherapy arm had a follow-up HSCT.
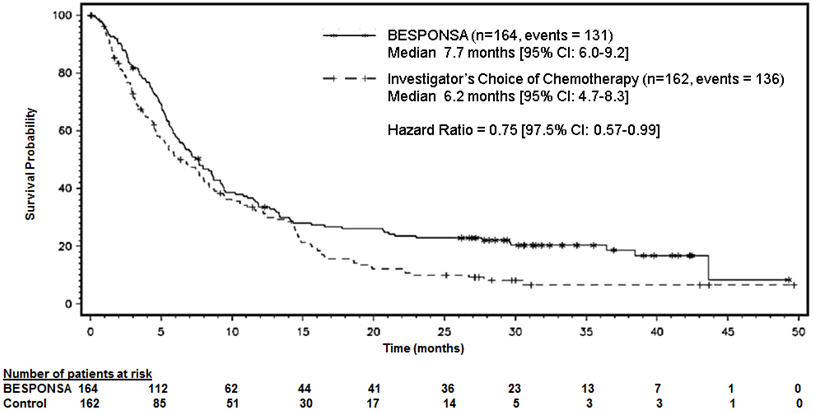
Figure 1 shows the analysis of overall survival (OS). The analysis of OS did not meet the pre-specified boundary for statistical significance.
Figure 1. Kaplan-Meier Curve for Overall Survival (Intent-to-Treat Population)
- 15. REFERENCES
-
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
BESPONSA (inotuzumab ozogamicin) for Injection is supplied as a white to off-white lyophilized powder in a single-dose vial for reconstitution and further dilution. Each vial delivers 0.9 mg inotuzumab ozogamicin. Each carton (NDC: 0008-0100-01) contains one single-dose vial.
-
17. PATIENT COUNSELING INFORMATION
Hepatotoxicity, Including Hepatic Veno-occlusive Disease (VOD) (also known as Sinusoidal Obstruction Syndrome)
Inform patients that liver problems, including severe, life-threatening, or fatal VOD, and increases in liver tests may develop during BESPONSA treatment. Inform patients that they should seek immediate medical advice if they experience symptoms of VOD, which may include elevated bilirubin, rapid weight gain, and abdominal swelling that may be painful. Inform patients that they should carefully consider the benefit/risk of BESPONSA treatment if they have a prior history of VOD or serious ongoing liver disease [see Warnings and Precautions (5.1)].
Increased Risk of Post-HSCT Non-Relapse Mortality
Inform patients that there is an increased risk of post-HSCT non-relapse mortality after receiving BESPONSA, that the most common causes of post-HSCT non-relapse mortality included infection and VOD. Advise patients to report signs and symptoms of infection [see Warnings and Precautions (5.2)].
Myelosuppression
Inform patients that decreased blood counts, which may be life-threatening, may develop during BESPONSA treatment and that complications associated with decreased blood counts may include infections, which may be life-threatening or fatal, and bleeding/hemorrhage events. Inform patients that signs and symptoms of infection, bleeding/hemorrhage, or other effects of decreased blood counts should be reported during treatment with BESPONSA [see Warnings and Precautions (5.3)].
Infusion Related Reactions
Advise patients to contact their health care provider if they experience symptoms such as fever, chills, rash, or breathing problems during the infusion of BESPONSA [see Warnings and Precautions (5.4)].
QT Interval Prolongation
Inform patients of symptoms that may be indicative of significant QTc prolongation including dizziness, lightheadedness, and syncope. Advise patients to report these symptoms and the use of all medications to their healthcare provider [see Warnings and Precautions (5.5)].
Embryo-Fetal Toxicity
Advise males and females of reproductive potential to use effective contraception during BESPONSA treatment and for at least 5 and 8 months after the last dose, respectively [see Use in Specific Populations (8.3)]. Advise females of reproductive potential to avoid becoming pregnant while receiving BESPONSA. Advise women to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, during treatment with BESPONSA. Inform the patient of the potential risk to the fetus [see Warnings and Precautions (5.7), Use in Specific Populations (8.1)].
Lactation
Advise women against breastfeeding while receiving BESPONSA and for 2 months after the last dose [see Use in Specific Populations (8.2)].
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 0.9 mg Vial Label
Pfizer
NDC: 0008-0100-01
Rx onlyBESPONSA™
(inotuzumab ozogamicin)
for Injection0.9 mg/vial
For Intravenous Infusion Only
No Preservatives
Single-dose vial.
Discard unused portion.
- PRINCIPAL DISPLAY PANEL - 0.9 mg Vial Carton
-
INGREDIENTS AND APPEARANCE
BESPONSA
inotuzumab ozogamicin injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0008-0100 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INOTUZUMAB OZOGAMICIN (UNII: P93RUU11P7) (INOTUZUMAB OZOGAMICIN - UNII:P93RUU11P7) INOTUZUMAB OZOGAMICIN 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) SUCROSE (UNII: C151H8M554) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0008-0100-01 1 in 1 CARTON 08/18/2017 1 4 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761040 08/18/2017 Labeler - Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc. (113008515) Establishment Name Address ID/FEI Business Operations Pharmacia and Upjohn Company LLC 618054084 LABEL(0008-0100) , PACK(0008-0100) Establishment Name Address ID/FEI Business Operations Wyeth BioPharma Division of Wyeth Pharmaceuticals LLC 174350868 ANALYSIS(0008-0100) , API MANUFACTURE(0008-0100) Establishment Name Address ID/FEI Business Operations Wyeth Pharmaceutical Division of Wyeth Holdings LLC 054065909 ANALYSIS(0008-0100) , API MANUFACTURE(0008-0100) , MANUFACTURE(0008-0100)
Trademark Results [Besponsa]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BESPONSA 86916876 5857763 Live/Registered |
Pfizer Inc. 2016-02-23 |
 BESPONSA 86391710 5003972 Live/Registered |
PFIZER INC. 2014-09-11 |
 BESPONSA 85390318 not registered Dead/Abandoned |
Pfizer Inc. 2011-08-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.