CUTIVATE- fluticasone propionate lotion
Cutivate by
Drug Labeling and Warnings
Cutivate by is a Prescription medication manufactured, distributed, or labeled by PharmaDerm a division of Fougera Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CUTIVATE® Lotion safely and effectively. See full prescribing information for CUTIVATE® Lotion.
CUTIVATE® (fluticasone propionate) Lotion, 0.05%, for topical use Initial U.S. Approval: 1990INDICATIONS AND USAGE
CUTIVATE® Lotion is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of atopic dermatitis in patients 3 months of age and older. (1)
DOSAGE AND ADMINISTRATION
- Apply a thin film to the affected skin areas once daily. Rub in gently. (2)
- Discontinue use when control is achieved. (2)
- Reassess diagnosis if no improvement in 2 weeks. (2)
- The safety and efficacy of CUTIVATE® Lotion have not been established beyond four weeks of use. (2)
- Avoid use under occlusion or application to diaper area. (2)
- Not for ophthalmic, oral, or intravaginal use. (2)
DOSAGE FORMS AND STRENGTHS
- Lotion, 0.05%, supplied in 120 mL bottles. (3)
CONTRAINDICATIONS
- None (4)
WARNINGS AND PRECAUTIONS
- Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression: Reversible HPA axis suppression and resulting glucocorticoid insufficiency can occur during or after withdrawal of treatment. Risk factors include the use of high-potency topical corticosteroids, use over large surface area, prolonged use, use under occlusion, concomitant use with other corticosteroid-containing products, altered skin barrier, liver failure, and use in pediatric patients. Minimize risk by mitigating the risk factors and use product as recommended. Modify use if HPA axis suppression is suspected. (5.1 , 8.4)
- Skin Irritation and Sensitization: CUTIVATE® Lotion contains the excipient imidurea which releases formaldehyde as a breakdown product. Formaldehyde may cause allergic sensitization or irritation upon contact with the skin. Discontinue use if irritation or sensitization develops. (5.2)
ADVERSE REACTIONS
- The most common adverse reactions (2%) were burning/stinging at the application site. (6.1)
- To report SUSPECTED ADVERSE REACTIONS, contact PharmaDerm®, A division of Fougera Pharmaceuticals Inc. at 1-800-645-9833 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression and Other Adverse Endocrine Effects
5.2 Local Adverse Reactions
5.3 Concomitant Skin Infections
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience: Controlled Clinical Trials
6.2 Clinical Trials Experience: Pediatric Open Label Trials
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Apply a thin film of CUTIVATE® Lotion to the affected skin areas once daily. Rub in gently.
Discontinue use when control is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary.
The safety and efficacy of CUTIVATE® Lotion have not been established beyond 4 weeks of use.
Avoid use with occlusive dressings or application to the diaper area [see Warnings and Precautions (5.1) and (5.2)].
CUTIVATE® Lotion is for topical use only, and not for ophthalmic, oral, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression and Other Adverse Endocrine Effects
Topical corticosteroids, including CUTIVATE® Lotion, can produce reversible HPA axis suppression with the potential for glucocorticoid insufficiency. Risk factors that predispose to HPA axis suppression include the use of high-potency topical corticosteroids, large treatment surface areas, prolonged use, use under occlusion, concomitant use of more than one corticosteroid-containing product, altered skin barrier, and liver failure. Pediatric patients may be at greater risk of HPA axis suppression due to their higher skin surface area to body mass ratios [see Use in Specific Populations (8.4)].
HPA axis suppression may occur during or after withdrawal of treatment. If HPA axis suppression is suspected, gradually withdraw the drug, reduce the frequency of application, or substitute a less potent topical corticosteroid. Evaluation of HPA axis suppression may be done by using the cosyntropin stimulation test.
The effects of CUTIVATE® Lotion on HPA axis function in pediatric patients were investigated in two trials. Among a total of 89 evaluable subjects from the two trials who were treated with CUTIVATE® Lotion twice daily for 3 to 4 weeks, a single subject with >90% body surface area treated showed laboratory evidence of transient suppression immediately post-treatment. The post cosyntropin stimulation test serum cortisol returned to a normal level (22.1µg/dL) within one week of the final treatment visit [see Use In Specific Populations (8.4) and Clinical Pharmacology (12.2)].
Cushing’s syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids.
Use of more than one corticosteroid-containing product at the same time may increase the total systemic absorption of topical corticosteroids.
5.2 Local Adverse Reactions
CUTIVATE® Lotion may cause local adverse reactions, including skin atrophy [see Adverse Reactions (6.1 , 6.2)]. The risk is greater with use under occlusion and with higher potency products.
CUTIVATE® Lotion contains the excipient imidurea which releases formaldehyde as a breakdown product. Formaldehyde may cause allergic sensitization or irritation upon contact with the skin. Avoid using CUTIVATE® Lotion in individuals with hypersensitivity to formaldehyde as it may prevent healing or worsen dermatitis.
If irritation develops, discontinue CUTIVATE® Lotion and institute appropriate therapy.
Allergic Contact Dermatitis
Allergic contact dermatitis with corticosteroids is usually diagnosed by observing a failure to heal rather than noticing a clinical exacerbation. Such an observation can be corroborated with appropriate diagnostic patch testing. Discontinue CUTIVATE® Lotion if appropriate. -
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- HPA Axis Suppression and Other Adverse Endocrine Effects [see Warnings and Precautions (5.1)]
- Local Adverse Reactions [see Warnings and Precautions (5.2)]
- Concomitant Skin Infections [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience: Controlled Clinical Trials
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In 2 multicenter vehicle-controlled clinical trials of once-daily application of CUTIVATE® Lotion by 196 adult and 242 pediatric patients, the total incidence of adverse reactions considered drug related by investigators was approximately 4%. These were local cutaneous reactions, usually mild and self-limiting, and consisted primarily of burning/stinging (2%). All other drug-related events occurred with an incidence of less than 1%, and included were contact dermatitis, exacerbation of atopic dermatitis, folliculitis of legs, pruritus, pustules on arm, rash, and skin infection. See Table 1.
The incidence of adverse reactions between the 242 pediatric subjects (age 3 months to < 17 years) and 196 adult subjects (17 years or older) was similar (4% and 5%, respectively).
Table 1: Adverse Reactions from Controlled Clinical Trials (n=438) ADVERSE REACTIONS
CUTIVATE® Lotion
VEHICLE
n=221
n=217
Burning/Stinging skin
4 (2%)
3 (1%)
Contact Dermatitis
0
1 (<1%)
Exacerbation of Atopic dermatitis
0
1 (<1%)
Folliculitis of legs
2 (<1%)
0
Irritant Contact Dermatitis
0
1 (<1%)
Pruritus
1 (<1%)
1 (<1%)
Pustules on Arms
1 (<1%)
0
Rash
1 (<1%)
2 (<1%)
Skin Infection
0
3 (1%)
During the clinical trials, eczema herpeticum occurred in a 33-year old male patient treated with CUTIVATE® Lotion.
Table 2 summarizes all adverse events by body system that occurred in at least 1% of patients in either the drug or vehicle group in the phase 3 controlled clinical trials.
Table 2: Adverse Events Occurring in ≥ 1% of Patients from Either Arm from Controlled Clinical Trials (n=438) Body System
CUTIVATE® Lotion
Vehicle Lotion
n=221
n=217
Any Adverse Event
77 (35%)
82 (38%)
Skin
Burning and Stinging
4 (2%)
3 (1%)
Pruritus
3 (1%)
5 (2%)
Rash
2 (<1%)
3 (1%)
Skin Infection
0
3 (1%)
Ear, Nose, Throat
Common Cold
9 (4%)
5 (2%)
Ear Infection
3 (1%)
3 (1%)
Nasal Sinus Infection
2 (<1%)
4 (2%)
Rhinitis
1 (<1%)
3 (1%)
Upper Respiratory
Tract Infection6 (3%)
7 (3%)
Gastrointestinal
Normal Tooth Eruption
2 (< 1%)
3 (1%)
Diarrhea
3 (1%)
0
Vomiting
3 (1%)
2 (<1%)
Lower Respiratory
Cough
7 (3%)
6 (3%)
Influenza
5 (2%)
0
Wheeze
0
3 (1%)
Neurology
Headache
4 (2%)
5 (2%)
Non-Site Specific
Fever
8 (4%)
8 (4%)
Seasonal Allergy
2 (<1%)
3 (1%)
6.2 Clinical Trials Experience: Pediatric Open Label Trials
In an open label HPA axis suppression trial of 44 pediatric subjects (ages ≥3 months to ≤6 years) CUTIVATE® Lotion was applied twice daily (rather than the indicated dosing regimen of once daily) to at least 35% of body surface area for 3 or 4 weeks. Subjects whose lesions cleared after 2 or 3 weeks of treatment continued to apply CUTIVATE® Lotion for an additional week. The overall incidence of adverse reactions was 14%. These were local, cutaneous reactions and included dry skin (7%), stinging at application site (5%), and excoriation (2%). Additionally, a 4-month-old patient treated with CUTIVATE® Lotion had marked elevations of the hepatic enzymes AST and ALT. [See Use in Specific Populations (8.4)]
In another open label HPA axis suppression trial in which CUTIVATE® Lotion was also applied twice daily (rather than the indicated dosing regimen of once daily), 56 pediatric subjects (ages ≥3 months to 12 months), were enrolled [see Use in Specific Populations (8.4)].
The adverse reactions included 2 cases of Herpes simplex at the application site (3.6%) and 3 cases of bacterial skin infections (5.4%).
6.3 Postmarketing Experience
The following local adverse reactions have been identified during post-approval use of CUTIVATE® Lotion: erythema, edema/swelling, and bleeding.
The following systemic adverse reactions have been identified during post-approval use of CUTIVATE® Cream and CUTIVATE® Ointment: immunosuppression/Pneumocystis jirovecii pneumonia/leukopenia/thrombocytopenia; hyperglycemia/ glycosuria; Cushing syndrome; generalized body edema/blurred vision; and acute urticarial reaction (edema, urticaria, pruritus, and throat swelling).
The following local adverse reactions have also been reported with the use of topical corticosteroids, and they may occur more frequently with the use of occlusive dressings or higher potency corticosteroids. These reactions include: acneiform eruptions, hypopigmentation, perioral dermatitis, skin atrophy, striae, hypertrichosis and miliaria.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Therefore, CUTIVATE® Lotion should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Systemic embryofetal development studies were conducted in mice, rats and rabbits.
Subcutaneous doses of 15, 45 and 150 μg/kg/day of fluticasone propionate were administered to pregnant female mice from gestation days 6 to 15. A teratogenic effect characteristic of corticosteroids (cleft palate) was noted after administration of 45 and 150 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 15 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Subcutaneous doses of 10, 30 and 100 μg/kg/day of fluticasone propionate were administered to pregnant female rats in two embryofetal development studies (one study administered fluticasone propionate from gestation days 6 to 15 and the other study from gestation days 7 to 17). In the presence of maternal toxicity, fetal effects noted at 100 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) included decreased fetal weights, omphalocele, cleft palate, and retarded skeletal ossification. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 10 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Subcutaneous doses of 0.08, 0.57 and 4 μg/kg/day of fluticasone propionate were administered to pregnant female rabbits from gestation days 6 to 18. Fetal effects noted at 4 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) included decreased fetal weights, cleft palate and retarded skeletal ossification. No treatment related effects on embryofetal toxicity or teratogenicity were noted at 0.57 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
Oral doses of 3, 30 and 300 μg/kg/day fluticasone propionate were administered to pregnant female rabbits from gestation days 8 to 20. No fetal or teratogenic effects were noted at oral doses up to 300 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study. However, no fluticasone propionate was detected in the plasma in this study, consistent with the established low bioavailability following oral administration.
Fluticasone propionate crossed the placenta following administration of a subcutaneous or an oral dose of 100 μg/kg tritiated fluticasone propionate to pregnant rats.
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and can suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when CUTIVATE® Lotion is administered to a nursing woman.
8.4 Pediatric Use
CUTIVATE® Lotion may be used in pediatric patients as young as 3 months of age. The safety and effectiveness of CUTIVATE® Lotion in pediatric patients below 3 months of age have not been established.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of systemic effects when treated with topical drugs. They are, therefore, also at greater risk of HPA axis suppression and adrenal insufficiency upon the use of topical corticosteroids [see Warnings and Precautions (5.1)].
In an HPA axis suppression trial, none of the 40 evaluable pediatric subjects, 4 months old to < 6 years old, with moderate to severe atopic dermatitis covering ≥ 35% Body Surface Area (BSA) who were treated with an exaggerated dosing regimen (twice daily) of CUTIVATE® Lotion experienced adrenal suppression (defined as a 30-minute post-stimulation cortisol level ≤18 micrograms/dL) [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].
In another HPA axis suppression trial, one of 49 (2%) evaluable pediatric subjects, 3 months to 11 months old, with moderate to severe atopic dermatitis covering ≥ 35% Body Surface Area (BSA) who applied an exaggerated dosing regimen (twice daily) of CUTIVATE® Lotion experienced reversible adrenal suppression (defined as a 30-minute post-stimulation cortisol level ≤18 micrograms/dL) following 4 weeks of therapy [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].
Systemic effects such as Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients, especially those with prolonged exposure to large doses of high-potency topical corticosteroids, or concomitant use of more than one corticosteroid product.
Local adverse reactions including skin atrophy have also been reported with use of topical corticosteroids in pediatric patients.
Parents of pediatric patients should be advised not to use this medication in the treatment of diaper dermatitis unless directed by a physician. CUTIVATE® Lotion should not be applied in the diaper areas as diapers or plastic pants may constitute occlusive dressing.
8.5 Geriatric Use
A limited number of patients above 65 years of age have been treated with CUTIVATE® Lotion in US and non-US clinical trials. Specifically only 8 patients above 65 years of age were treated with CUTIVATE® Lotion in controlled clinical trials. The number of patients is too small to permit separate analyses of efficacy and safety.
-
11 DESCRIPTION
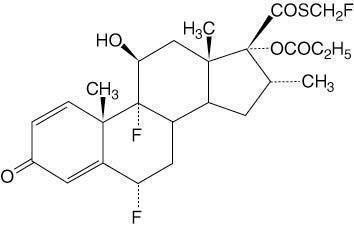
CUTIVATE® (fluticasone propionate) Lotion USP, 0.05% contains fluticasone propionate, USP [S-Fluoromethyl 6α, 9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbothioate], a synthetic fluorinated corticosteroid, for topical use. The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
Chemically, fluticasone propionate, USP is C25H31F3O5S. It has the following structural formula:
Fluticasone propionate, USP has a molecular weight of 500.6. It is a white to off-white powder and is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
Each gram of CUTIVATE® Lotion USP contains 0.5 mg fluticasone propionate, USP in a white to off white lotion base of cetomacrogol 1000, cetostearyl alcohol, citric acid monohydrate, dimethicone 350, imidurea, isopropyl myristate, methylparaben, propylene glycol, propylparaben, purified water, and sodium citrate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action of CUTIVATE® Lotion in atopic dermatitis is unknown.
12.2 Pharmacodynamics
Vasoconstrictor Assay
Trials performed with CUTIVATE® Lotion indicate that it is in the medium range of potency as demonstrated in vasoconstrictor trials in healthy subjects when compared with other topical corticosteroids. However, similar blanching scores do not necessarily imply therapeutic equivalence.
Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression
In an open label HPA axis suppression trial (Trial A), 42 pediatric subjects (ages 4 months to <6 years) with moderate to severe atopic dermatitis covering ≥ 35% Body Surface Area (BSA) who were treated with an exaggerated dosing regimen of CUTIVATE® Lotion twice daily (rather than the indicated dosing regimen of once daily) for at least 3 to 4 weeks were assessed for HPA axis suppression. The mean BSA treated was 65%. None of the 40 evaluable subjects were suppressed. The criterion for HPA axis suppression was a serum cortisol level of less than or equal to 18 micrograms per deciliter at 30-minutes after cosyntropin stimulation.
Another open label HPA axis suppression trial (Trial B) enrolled 56 pediatric subjects (ages 3 months to 11 months) with moderate to severe atopic dermatitis covering ≥ 35% BSA. Subjects were treated with an exaggerated dosing regimen for of CUTIVATE® Lotion twice daily over a period of 3 or 4 weeks. The mean BSA treated was 54%. Out of 56 subjects, 49 were considered evaluable with respect to their adrenal axis function post-treatment. One of 49 subjects showed laboratory evidence of suppression immediately post treatment. The criterion for HPA axis suppression was a serum cortisol level of less than or equal to 18 micrograms per deciliter at 30-minutes after cosyntropin stimulation. Repeated test one week later showed the post cosyntropin stimulation testing serum cortisol returned to normal level (22.1 µg/dL). This 4-month old subject had a baseline treatment BSA of 94% and was reported to have received 100% of the twice-daily applications of CUTIVATE® Lotion over the 27 day treatment period.
12.3 Pharmacokinetics
Absorption
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressing enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption.Plasma fluticasone levels were measured in a subset of subjects 2 to 5 years and 11 months of age in HPA axis suppression trial (Trial A) described above. A total of 13 (62%) of 21 subjects tested had measurable fluticasone at the end of 3 to 4 weeks of treatment. The mean ± SD fluticasone plasma concentration was 0.16 ± 0.23 ng/mL. Three subjects aged 3, 4, and 4 years had fluticasone concentrations over 0.30 ng/mL, with one of them having a concentration of 0.82 ng/mL. No data were obtained for subjects < 2 years of age.
Distribution
The percentage of fluticasone propionate bound to human plasma proteins averaged 91%. Fluticasone propionate is weakly and reversibly bound to erythrocytes. Fluticasone propionate is not significantly bound to human transcortin.Metabolism
No metabolites of fluticasone propionate were detected in an in vitro study of radiolabeled fluticasone propionate incubated in a human skin homogenate.Fluticasone propionate is metabolized in the liver by cytochrome P450 3A4-mediated hydrolysis of the 5-fluoromethyl carbothiolate grouping. This transformation occurs in 1 metabolic step to produce the inactive 17β-carboxylic acid metabolite, the only known metabolite detected in man. This metabolite has approximately 2000 times less affinity than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In an oral (gavage) mouse carcinogenicity study, doses of 0.1, 0.3 and 1 mg/kg/day fluticasone propionate were administered to mice for 18 months. Fluticasone propionate demonstrated no tumorigenic potential at oral doses up to 1 mg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study.
In a dermal mouse carcinogenicity study, 0.05% fluticasone propionate ointment (40 μl) was topically administered for 1, 3 or 7 days/week for 80 weeks. Fluticasone propionate demonstrated no tumorigenic potential at dermal doses up to 6.7 μg/kg/day (less than the MRHD in adults based on body surface area comparisons) in this study.
Fluticasone propionate revealed no evidence of mutagenic or clastogenic potential based on the results of five in vitro genotoxicity tests (Ames assay, E. coli fluctuation test, S. cerevisiae gene conversion test, Chinese hamster ovary cell chromosome aberration assay and human lymphocyte chromosome aberration assay) and one in vivo genotoxicity test (mouse micronucleus assay).
No evidence of impairment of fertility or effect on mating performance was observed in a fertility and general reproductive performance study conducted in male and female rats at subcutaneous doses up to 50 μg/kg/day (less than the MRHD in adults based on body surface area comparisons).
-
14 CLINICAL STUDIES
CUTIVATE® Lotion applied once daily was superior to vehicle in the treatment of atopic dermatitis in two clinical trials. The two trials enrolled 438 subjects with atopic dermatitis aged 3 months and older, of which 169 subjects were selected as having clinically significant signs of erythema, infiltration/papulation, and erosion/oozing/crusting at baseline. Clinically significant was defined as having moderate or severe involvement for at least two of the three signs (erythema, infiltrations/papulation, or erosion/oozing/crusting), in at least 2 body regions. Subjects who had moderate to severe disease in a single body region were excluded from the analysis.
Table 3 presents the percentage of subjects who completely cleared of erythema, infiltration/papulation and erosion/oozing/crusting at Week 4 out of those subjects with clinically significant baseline signs.
Table 3: Complete Clearance Rate: For Patients with Clinically Significant Signs at Baseline CUTIVATE® Lotion
Vehicle
Study 1
9/45 (20%)
0/37 (0%)
Study 2
7/44 (16%)
1/43 (2%)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
CUTIVATE® (fluticasone propionate) Lotion USP, 0.05% is white to off-white in color, and supplied as follows:
120 mL bottle NDC: 10337-434-04
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration Instructions
Advise the patient of appropriate CUTIVATE® Lotion administration instructions, including those that will mitigate HPA-Axis suppression [see Warnings and Precautions (5.1)] and local adverse reactions [see Warnings and Precautions (5.2)]:- Discontinue therapy when control is achieved in less than 4 weeks; if no improvement is seen within 2 weeks, contact the healthcare provider.
- Avoid contact with the eyes.
- Do not bandage the treated skin area, or cover or wrap it to cause occlusion unless directed by the healthcare provider.
- Do not use CUTIVATE® Lotion in the treatment of diaper dermatitis unless directed by the healthcare provider, as diapers or plastic pants may constitute occlusive dressing and enhance absorption.
- Do not use on the face, underarms, or groin areas unless directed by the healthcare provider.
Local Adverse Reactions
Advise the patient to report any signs of local adverse reactions to their healthcare provider [see Warnings and Precautions (5.2)].- Advise patients to report to their healthcare provider if they are allergic to formaldehyde.
- SPL UNCLASSIFIED SECTION
-
PATIENT INFORMATION CUTIVATE® (CUE' ti vāt) (fluticasone propionate) Lotion USP, 0.05%
Important: CUTIVATE® Lotion is for use on skin only (topical). Do not get CUTIVATE® Lotion near or in your eyes, mouth, or vagina.
Read this Patient Information before you start using CUTIVATE® Lotion and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is CUTIVATE® Lotion?
CUTIVATE® Lotion is a prescription corticosteroid medicine used on the skin (topical) for the relief of inflammation and itching caused by certain skin conditions, including atopic dermatitis and eczema in people 3 months of age and older.
It is not known if CUTIVATE® Lotion is safe and effective in children under 3 months of age.Before using CUTIVATE® Lotion, tell your healthcare provider about all of your medical conditions, including if you:
- have a skin infection at the site to be treated. You may also need medicine to treat the skin infection.
- have adrenal gland problems
- have liver problems
- have diabetes
- have thinning skin (atrophy) at the site to be treated
- are allergic to formaldehyde. If you are allergic to formaldehyde, CUTIVATE® Lotion may cause skin irritation, prevent your skin from healing or worsen your skin condition.
- are pregnant or plan to become pregnant. It is not known if CUTIVATE® Lotion will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if CUTIVATE® Lotion can pass into your breast milk and harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take other corticosteroid medicines by mouth or use other products on your skin that contain corticosteroids.
How should I use CUTIVATE® Lotion?
- Use CUTIVATE® Lotion exactly as your healthcare provider tells you to use it.
- Apply a thin film of CUTIVATE® Lotion to the affected area 1 time each day. Gently rub into your skin.
- Do not bandage, cover, or wrap the treated area unless your healthcare provider tells you to. Do not apply CUTIVATE® Lotion to dermatitis in the diaper area unless your healthcare provider tells you to.
- Wash your hands after applying CUTIVATE® Lotion, unless your hands are being treated.
- Tell your healthcare provider if your symptoms get worse with CUTIVATE® Lotion or if your symptoms do not improve after 2 weeks of treatment. It is not known if CUTIVATE® Lotion is safe or effective when used more than 4 weeks.
What are possible side effects with CUTIVATE® Lotion?
CUTIVATE® Lotion may cause serious side effects, including:- CUTIVATE® Lotion can pass through your skin and may cause adrenal gland problems. This is more likely to happen if you use CUTIVATE® Lotion for too long, use it over a large treatment area, use it with other topical medicines that contain corticosteroids, cover the treated area, or have liver failure. Your healthcare provider may do blood tests to check your adrenal gland function during and after treatment with CUTIVATE® Lotion.
- Skin problems, including skin reactions or thinning of your skin (atrophy), skin infections, and allergic reactions (allergic contact dermatitis) at the treatment site. Tell your healthcare provider if you get any skin reactions such as pain, tenderness, swelling, or healing problems.
The most common side effects of CUTIVATE® Lotion include burning and stinging at the treatment site.
These are not all the possible side effects with CUTIVATE® Lotion. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store CUTIVATE® Lotion?
- Store CUTIVATE® Lotion between 59°F to 86°F (15°C to 30°C).
- Do not refrigerate.
- Keep the bottle tightly closed.
Keep CUTIVATE® Lotion and all medicines out of the reach of children.
General information about the safe and effective use of CUTIVATE® Lotion.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use CUTIVATE® Lotion for a condition for which it was not prescribed. Do not give CUTIVATE® Lotion to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about CUTIVATE® Lotion that is written for health professionals.What are the ingredients in CUTIVATE® Lotion?
Active ingredient: fluticasone propionate, USP
Inactive ingredients: cetomacrogol 1000, cetostearyl alcohol, citric acid monohydrate, dimethicone 350, imidurea, isopropyl myristate, methylparaben, propylene glycol, propylparaben, purified water, and sodium citrateManufactured by:

For more information, go to www.pharmaderm.com or call 1-800-645-9833.
This Patient Information has been approved by the U.S. Food and Drug Administration46154831C/46155409C
Revised: 08/2018
#192
-
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 120 mL LABEL
PharmaDerm® NDC: 10337-434-04
CUTIVATE®
(fluticasone propionate)
Lotion, 0.05%For topical use only –
Not for ophthalmic, oral
or intravaginal use.Rx only
120 mL
-
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 120 mL CARTON
PharmaDerm® NDC: 10337-434-04
CUTIVATE®
(fluticasone propionate)
Lotion, 0.05%For topical use only –
Not for ophthalmic, oral
or intravaginal use.Rx only
120 mL
-
INGREDIENTS AND APPEARANCE
CUTIVATE
fluticasone propionate lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10337-434 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength fluticasone propionate (UNII: O2GMZ0LF5W) (fluticasone - UNII:CUT2W21N7U) fluticasone propionate 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETETH-20 (UNII: I835H2IHHX) Cetostearyl Alcohol (UNII: 2DMT128M1S) DIMETHICONE (UNII: 92RU3N3Y1O) Imidurea (UNII: M629807ATL) Isopropyl Myristate (UNII: 0RE8K4LNJS) Methylparaben (UNII: A2I8C7HI9T) Propylene Glycol (UNII: 6DC9Q167V3) propylparaben (UNII: Z8IX2SC1OH) Water (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10337-434-07 7 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/05/2009 2 NDC: 10337-434-66 6 in 1 CARTON 10/05/2009 2 7 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 10337-434-04 1 in 1 CARTON 10/05/2009 3 120 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021152 10/05/2009 Labeler - PharmaDerm a division of Fougera Pharmaceuticals Inc. (043838424)
Trademark Results [Cutivate]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CUTIVATE 78181823 2986108 Live/Registered |
FOUGERA PHARMACEUTICALS INC. 2002-11-05 |
 CUTIVATE 74634377 not registered Dead/Abandoned |
Glaxo Group Limited 1995-02-15 |
 CUTIVATE 73826831 1653425 Dead/Cancelled |
GLAXO GROUP LIMITED 1989-09-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.