zegerid with magnesium hydroxide- omeprazole, sodium bicarbonate and magnesium hydroxide tablet, chewable
Drug Labeling and Warnings
Drug Details [pdf]
- N/A - Section Title Not Found In Database
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
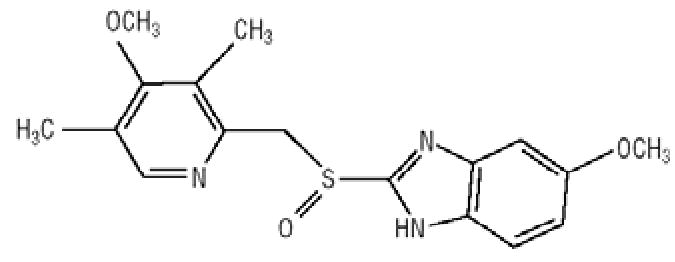
ZEGERID® with Magnesium Hydroxide (omeprazole/sodium bicarbonate/magnesium hydroxide) is a combination of omeprazole, a proton-pump inhibitor, and sodium bicarbonate plus magnesium hydroxide, both of which are antacids. Omeprazole is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, a racemic mixture of two enantiomers that inhibits gastric acid secretion. Its empirical formula is C17H19N3O3S, with a molecular weight of 345.42. The structural formula is:
Omeprazole is a white to off-white crystalline powder which melts with decomposition at about 155°C. It is a weak base, freely soluble in ethanol and methanol, and slightly soluble in acetone and isopropanol and very slightly soluble in water. The stability of omeprazole is a function of pH; it is rapidly degraded in acid media, but has acceptable stability under alkaline conditions.
ZEGERID with Magnesium Hydroxide is available in two strengths, 40 mg and 20 mg of omeprazole, and is formulated as an immediate-release chewable tablet. Each chewable tablet contains either 40 mg or 20 mg of omeprazole and 600 mg of sodium bicarbonate plus 700 mg of magnesium hydroxide with the following inactive ingredients: hydroxypropyl cellulose, croscarmellose sodium, xylitol, sucralose, flavoring, magnesium stearate, and FD&C Red #40 Aluminum Lake.
-
CLINICAL PHARMACOLOGY
Omeprazole is acid labile and thus rapidly degraded by gastric acid. ZEGERID with Magnesium Hydroxide is an immediate-release chewable tablet formulation that contains an antacid component (sodium bicarbonate plus magnesium hydroxide) which raises the gastric pH and thus protects omeprazole from acid degradation.
Pharmacokinetics:
Absorption
When ZEGERID with Magnesium Hydroxide chewable tablets are administered on an empty stomach at least 1 hour prior to a meal, the absorption of omeprazole is rapid, with a mean peak plasma level (%CV) of omeprazole being 1763 ng/mL (25%) and time to peak of approximately 30 minutes (range 10-90 min) after a single-dose or repeated-dose administration.
Following single or repeated once daily dosing, peak plasma concentrations of omeprazole from ZEGERID are approximately proportional from 20 to 40 mg doses of omeprazole, but a greater than linear mean AUC (three-fold increase) is observed when doubling the dose to 40 mg. The bioavailability of omeprazole from ZEGERID increases upon repeated administration.
When ZEGERID with Magnesium Hydroxide chewable tablets are administered 1 hour after a meal, the AUC is reduced by approximately 22% relative to administration 1 hour prior to a meal.
Metabolism
Following single dose oral administration of omeprazole, the majority of the dose (about 77%) is eliminated in urine as at least six metabolites. Two metabolites have been identified as hydroxyomeprazole and the corresponding carboxylic acid. The remainder of the dose was recoverable in feces. This implies a significant biliary excretion of the metabolites of omeprazole. Three metabolites have been identified in plasma – the sulfide and sulfone derivatives of omeprazole, and hydroxyomeprazole. These metabolites have very little or no antisecretory activity.
Special Populations
Geriatric
The elimination rate of omeprazole was somewhat decreased in the elderly, and bioavailability was increased. Omeprazole was 76% bioavailable when a single 40-mg oral dose of omeprazole (buffered solution) was administered to healthy elderly subjects, versus 58% in young subjects given the same dose. Nearly 70% of the dose was recovered in urine as metabolites of omeprazole and no unchanged drug was detected. The plasma clearance of omeprazole was 250 mL/min (about half that of young subjects) and its plasma half-life averaged one hour, similar to that of young healthy subjects.
Pediatric
The pharmacokinetics of ZEGERID with Magnesium Hydroxide have not been studied in patients < 18 years of age.
Gender
There are no known differences in the absorption or excretion of omeprazole between males and females.
Hepatic Insufficiency
In patients with chronic hepatic disease, the bioavailability of omeprazole from a buffered solution increased to approximately 100% compared to an I.V. dose, reflecting decreased first-pass effect, and the mean plasma half-life of the drug increased to nearly 3 hours compared to the mean half-life of 1 hour in normal subjects. Plasma clearance averaged 70 mL/min, compared to a value of 500-600 mL/min in normal subjects.
Renal Insufficiency
In patients with chronic renal impairment, whose creatinine clearance ranged between 10 and 62 mL/min/1.73 m2, the disposition of omeprazole from a buffered solution was very similar to that in healthy subjects, although there was a slight increase in bioavailability. Because urinary excretion is a primary route of excretion of omeprazole metabolites, their elimination slowed in proportion to the decreased creatinine clearance.
ZEGERID chewable tablets contain magnesium hydroxide (292 mg of Mg++); therefore, magnesium levels should be closely monitored when using this product in patients with renal failure.
Asians
In pharmacokinetic studies of single 20-mg omeprazole doses, an increase in AUC of approximately four-fold was noted in Asian subjects compared to Caucasians.
Dose adjustment, particularly where maintenance of healing of erosive esophagitis is indicated, for the hepatically impaired and Asian subjects should be considered.
Drug-Drug Interactions
When omeprazole 40 mg was given once daily in combination with clarithromycin 500 mg every 8 hours to healthy adult male subjects, the steady-state plasma concentrations of omeprazole were increased by the concomitant administration of clarithromycin [Cmax, AUC(0-24) and T½ increased 30%, 89%, and 34%, respectively].
Pharmacodynamics:
Mechanism of Action
Omeprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that do not exhibit anticholinergic or H2 histamine antagonistic properties, but that suppress gastric acid secretion by specific inhibition of the H+/K+ ATPase enzyme system at the secretory surface of the gastric parietal cell. Because this enzyme system is regarded as the acid (proton) pump within the gastric mucosa, omeprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose related and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus. Animal studies indicate that after rapid disappearance from plasma, omeprazole can be found within the gastric mucosa for a day or more.
Antisecretory Activity
Results from PK/PD studies of the antisecretory effect of repeated once-daily dosing of 40 mg and 20 mg of ZEGERID with Magnesium Hydroxide chewable tablets in healthy subjects are shown in Table 1 below.
Table 1: Effect of ZEGERID® with Magnesium Hydroxide Chewable Tablets on Intragastric pH on Day 7 Omeprazole/Sodium Bicarbonate/Magnesium Hydroxide Parameter 40 mg/600 mg/700 mg
(n = 35)20 mg/600 mg/700 mg
(n = 29)Note: Values are medians. All parameters were measured over a 24-hour period.
% Decrease from Baseline for Integrated Gastric Acidity (mmol*hr/L) 73% 72% Coefficient of variation 19% 28% % Time Gastric pH > 4
(Hours)62%
(14.9 h)57%
(13.8 h)Coefficient of variation 30% 32% Median pH 5.1 4.8 Coefficient of variation 24% 29% The antisecretory effect of omeprazole thus lasts far longer than would be expected from the very short (1 hour) plasma half-life, apparently due to irreversible binding to the parietal H+/K+ ATPase enzyme.
Repeated single daily oral doses of ZEGERID have produced nearly 100% inhibition of 24-hour integrated gastric acidity in some subjects.
Enterochromaffin-like (ECL) Cell Effects
In 24-month carcinogenicity studies in rats, a dose-related significant increase in gastric carcinoid tumors and ECL cell hyperplasia was observed in both male and female animals (see PRECAUTIONS, Carcinogenesis, Mutagenesis, Impairment of Fertility). Carcinoid tumors have also been observed in rats subjected to fundectomy or long-term treatment with other proton pump inhibitors or high doses of H2-receptor antagonists. Human gastric biopsy specimens have been obtained from more than 3000 patients treated with omeprazole in long-term clinical trials. The incidence of ECL cell hyperplasia in these studies increased with time; however, no case of ECL cell carcinoids, dysplasia, or neoplasia has been found in these patients. These studies are of insufficient duration and size to rule out the possible influence of long-term administration of omeprazole on the development of any premalignant or malignant conditions.
Serum Gastrin Effects
In studies involving more than 200 patients, serum gastrin levels increased during the first 1 to 2 weeks of once-daily administration of therapeutic doses of omeprazole in parallel with inhibition of acid secretion. No further increase in serum gastrin occurred with continued treatment. In comparison with histamine H2-receptor antagonists, the median increases produced by 20 mg doses of omeprazole were higher (1.3 to 3.6 fold vs. 1.1 to 1.8 fold increase). Gastrin values returned to pretreatment levels, usually within 1 to 2 weeks after discontinuation of therapy.
Other Effects
Systemic effects of omeprazole in the CNS, cardiovascular, and respiratory systems have not been found to date. Omeprazole, given in oral doses of 30 or 40 mg for 2 to 4 weeks, had no effect on thyroid function, carbohydrate metabolism, or circulating levels of parathyroid hormone, cortisol, estradiol, testosterone, prolactin, cholecystokinin, or secretin.
No effect on gastric emptying of the solid and liquid components of a test meal was demonstrated after a single dose of omeprazole 90 mg. In healthy subjects, a single I.V. dose of omeprazole (0.35 mg/kg) had no effect on intrinsic factor secretion. No systematic dose-dependent effect has been observed on basal or stimulated pepsin output in humans. However, when intragastric pH is maintained at 4.0 or above, basal pepsin output is low, and pepsin activity is decreased.
As do other agents that elevate intragastric pH, omeprazole administered for 14 days in healthy subjects produced a significant increase in the intragastric concentrations of viable bacteria. The pattern of the bacterial species was unchanged from that commonly found in saliva. All changes resolved within three days of stopping treatment.
The course of Barrett's esophagus in 106 patients was evaluated in a U.S. double-blind controlled study of omeprazole 40 mg b.i.d. for 12 months followed by 20 mg b.i.d. for 12 months or ranitidine 300 mg b.i.d. for 24 months. No clinically significant impact on Barrett's mucosa by antisecretory therapy was observed. Although neosquamous epithelium developed during antisecretory therapy, complete elimination of Barrett's mucosa was not achieved. No significant difference was observed between treatment groups in development of dysplasia in Barrett's mucosa and no patient developed esophageal carcinoma during treatment. No significant differences between treatment groups were observed in development of ECL cell hyperplasia, corpus atrophic gastritis, corpus intestinal metaplasia, or colon polyps exceeding 3 mm in diameter (see also CLINICAL PHARMACOLOGY, Enterochromaffin-like (ECL) Cell Effects).
Clinical Studies
Duodenal Ulcer Disease
Active Duodenal Ulcer – In a multicenter, double-blind, placebo controlled study of 147 patients with endoscopically documented duodenal ulcer, the percentage of patients healed (per protocol) at 2 and 4 weeks was significantly higher with omeprazole 20 mg once a day than with placebo (p ≤ 0.01). (See Table 2.)
Table 2: Treatment of Active Duodenal Ulcer; % of Patients Healed Omeprazole
20 mg a.m.
(n = 99)Placebo
a.m.
(n = 48)* (p ≤ 0.01)
Week 2 41* 13 Week 4 75* 27 Complete daytime and nighttime pain relief occurred significantly faster (p ≤ 0.01) in patients treated with omeprazole 20 mg than in patients treated with placebo. At the end of the study, significantly more patients who had received omeprazole had complete relief of daytime pain (p ≤ 0.05) and nighttime pain (p ≤ 0.01).
In a multicenter, double-blind study of 293 patients with endoscopically documented duodenal ulcer, the percentage of patients healed (per protocol) at 4 weeks was significantly higher with omeprazole 20 mg once a day than with ranitidine 150 mg b.i.d. (p < 0.01). (See Table 3.)
Table 3: Treatment of Active Duodenal Ulcer; % of Patients Healed Omeprazole
20 mg a.m.
(n = 145)Ranitidine
150 mg b.i.d.
(n = 148)* (p < 0.01)
Week 2 42 34 Week 4 82* 63 Healing occurred significantly faster in patients treated with omeprazole than in those treated with ranitidine 150 mg b.i.d. (p < 0.01).
In a foreign multinational randomized, double-blind study of 105 patients with endoscopically documented duodenal ulcer, 40 mg and 20 mg of omeprazole were compared to 150 mg b.i.d. of ranitidine at 2, 4 and 8 weeks. At 2 and 4 weeks both doses of omeprazole were statistically superior (per protocol) to ranitidine, but 40 mg was not superior to 20 mg of omeprazole, and at 8 weeks there was no significant difference between any of the active drugs. (See Table 4.)
Table 4: Treatment of Active Duodenal Ulcer; % of Patients Healed Omeprazole
40 mg
(n = 36)Omeprazole
20 mg
(n = 34)Ranitidine
150 mg b.i.d.
(n = 35)*(p ≤ 0.01)
Week 2 83* 83* 53 Week 4 100* 97* 82 Week 8 100 100 94 Gastric Ulcer
In a U.S. multicenter, double-blind, study of omeprazole 40 mg once a day, 20 mg once a day, and placebo in 520 patients with endoscopically diagnosed gastric ulcer, the following results were obtained. (See Table 5.)
Table 5: Treatment of Gastric Ulcer; % of Patients Healed (All Patients Treated) Omeprazole
40 mg q.d.
(n = 214)Omeprazole
20 mg q.d.
(n = 202)Placebo
(n = 104)** (p < 0.01) omeprazole 40 mg or 20 mg versus placebo
+ (p < 0.05) omeprazole 40 mg versus 20 mg
Week 4 55.6** 47.5** 30.8 Week 8 82.7**,+ 74.8** 48.1 For the stratified groups of patients with ulcer size less than or equal to 1 cm, no difference in healing rates between 40 mg and 20 mg was detected at either 4 or 8 weeks. For patients with ulcer size greater than 1 cm, 40 mg was significantly more effective than 20 mg at 8 weeks.
In a foreign, multinational, double-blind study of 602 patients with endoscopically diagnosed gastric ulcer, omeprazole 40 mg once a day, 20 mg once a day, and ranitidine 150 mg twice a day were evaluated. (See Table 6.)
Table 6: Treatment of Gastric Ulcer; % of Patients Healed (All Patients Treated) Omeprazole
40 mg q.d.
(n = 187)Omeprazole
20 mg q.d.
(n = 200)Ranitidine
150 mg b.i.d.
(n = 199)**(p < 0.01) Omeprazole 40 mg versus ranitidine
++(p < 0.01) Omeprazole 40 mg versus 20 mg
Week 4 78.1**,++ 63.5 56.3 Week 8 91.4**,++ 81.5 78.4 Gastroesophageal Reflux Disease (GERD)
Symptomatic GERD
A placebo controlled study was conducted in Scandinavia to compare the efficacy of omeprazole 20 mg or 10 mg once daily for up to 4 weeks in the treatment of heartburn and other symptoms in GERD patients without erosive esophagitis. Results are shown in Table 7.
Table 7: % Successful Symptomatic Outcomea Omeprazole
20 mg a.m.Omeprazole
10 mg a.m.Placebo
a.m.a Defined as complete resolution of heartburn
* (p < 0.005) versus 10 mg
† (p < 0.005) versus placebo
All patients 46*,† (n = 205) 31† (n = 199) 13 (n = 105) Patients with
confirmed GERD56*,† (n = 115) 36† (n = 109) 14 (n = 59) Erosive Esophagitis
In a U.S. multicenter double-blind placebo controlled study of 40 mg or 20 mg of omeprazole in patients with symptoms of GERD and endoscopically diagnosed erosive esophagitis of grade 2 or above, the percentage healing rates (per protocol) were as shown in Table 8.
Table 8: % Patients Healed Omeprazole
40 mg
(n = 87)Omeprazole
20 mg
(n = 83)Placebo
(n = 43)* (p < 0.01) Omeprazole versus placebo.
Week 4 45* 39* 7 Week 8 75* 74* 14 In this study, the 40-mg dose was not superior to the 20-mg dose of omeprazole in the percentage healing rate. Other controlled clinical trials have also shown that omeprazole is effective in severe GERD. In comparisons with histamine H2-receptor antagonists in patients with erosive esophagitis, grade 2 or above, omeprazole in a dose of 20 mg was significantly more effective than the active controls. Complete daytime and nighttime heartburn relief occurred significantly faster (p < 0.01) in patients treated with omeprazole than in those taking placebo or histamine H2-receptor antagonists.
In this and five other controlled GERD studies, significantly more patients taking 20 mg omeprazole (84%) reported complete relief of GERD symptoms than patients receiving placebo (12%).
Long Term Maintenance Treatment of Erosive Esophagitis
In a U.S. double-blind, randomized, multicenter, placebo controlled study, two dose regimens of omeprazole were studied in patients with endoscopically confirmed healed esophagitis. Results to determine maintenance of healing of erosive esophagitis are shown in Table 9.
Table 9: Life Table Analysis Omeprazole
20 mg q.d.
(n = 138)Omeprazole
20 mg
3 days per week
(n = 137)Placebo
(n = 131)* (p < 0.01) Omeprazole 20 mg q.d. versus Omeprazole 20 mg 3 consecutive days per week or placebo.
Percent in endoscopic remission at 6 months 70* 34 11 In an international multicenter double-blind study, omeprazole 20 mg daily and 10 mg daily were compared to ranitidine 150 mg twice daily in patients with endoscopically confirmed healed esophagitis. Table 10 provides the results of this study for maintenance of healing of erosive esophagitis.
Table 10: Life Table Analysis Omeprazole
20 mg q.d.
(n = 131)Omeprazole
10 mg q.d.
(n = 133)Ranitidine
150 mg b.i.d.
(n = 128)* (p = 0.01) Omeprazole 20 mg q.d. versus Omeprazole 10 mg q.d. or Ranitidine.
‡ (p = 0.03) Omeprazole 10 mg q.d. versus Ranitidine.
Percent in endoscopic remission at 12 months 77* 58‡ 46 In patients who initially had grades 3 or 4 erosive esophagitis, for maintenance after healing, 20 mg daily of omeprazole was effective, while 10 mg did not demonstrate effectiveness.
-
INDICATIONS AND USAGE
Duodenal Ulcer
ZEGERID with Magnesium Hydroxide is indicated for short-term treatment of active duodenal ulcer. Most patients heal within four weeks. Some patients may require an additional four weeks of therapy.
Gastric Ulcer
ZEGERID with Magnesium Hydroxide is indicated for short-term treatment (4-8 weeks) of active benign gastric ulcer. (See CLINICAL PHARMACOLOGY, Clinical Studies, Gastric Ulcer.)
Treatment of Gastroesophageal Reflux Disease (GERD)
Symptomatic GERD
ZEGERID with Magnesium Hydroxide is indicated for the treatment of heartburn and other symptoms associated with GERD.
Erosive Esophagitis
ZEGERID with Magnesium Hydroxide is indicated for the short-term treatment (4-8 weeks) of erosive esophagitis which has been diagnosed by endoscopy. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
The efficacy of ZEGERID with Magnesium Hydroxide used for longer than 8 weeks in these patients has not been established. In the rare instance of a patient not responding to 8 weeks of treatment, it may be helpful to give up to an additional 4 weeks of treatment. If there is recurrence of erosive esophagitis or GERD symptoms (eg, heartburn), additional 4-8 week courses of omeprazole may be considered.
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Symptomatic response to therapy with omeprazole does not preclude the presence of gastric malignancy.
Atrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with omeprazole.
Each 20 mg and 40 mg ZEGERID with Magnesium Hydroxide chewable tablet contains 600 mg (7 mEq) of sodium bicarbonate (equivalent to 164 mg of Na+) and 700 mg (24 mEq) of magnesium hydroxide (equivalent to 292 mg of Mg++).
The sodium content of this product should be taken into consideration when administering to patients on a sodium-restricted diet.
Magnesium hydroxide should be used with caution in neonates, elderly, and in patients with renal impairment or renal disease due to increased risk of developing hypermagnesemia and magnesium toxicity. Magnesium hydroxide should not be used in patients with renal failure unless serum magnesium levels are being closely monitored.
Sodium bicarbonate is contraindicated in patients with metabolic alkalosis and hypocalcemia. Sodium bicarbonate should be used with caution in patients with Bartter's syndrome, hypokalemia, respiratory alkalosis, and problems with acid-base balance. Long-term administration of bicarbonate with calcium or milk can cause milk-alkali syndrome.
Information for Patients
ZEGERID with Magnesium Hydroxide should be taken on an empty stomach at least one hour prior to a meal. ZEGERID with Magnesium Hydroxide chewable tablets are available in 40 mg and 20 mg dosage strengths of omeprazole with 600 mg sodium bicarbonate plus 700 mg magnesium hydroxide per tablet.
Directions for Use: DO NOT SWALLOW WHOLE AND DO NOT SUBSTITUTE FOR OTHER ZEGERID DOSAGE FORMS. Chew the tablet and swallow with water. DO NOT USE OTHER LIQUIDS.
Drug Interactions
Omeprazole can prolong the elimination of diazepam, warfarin and phenytoin, drugs that are metabolized by oxidation in the liver. There have been reports of increased INR and prothrombin time in patients receiving proton pump inhibitors, including omeprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with proton pump inhibitors and warfarin may need to be monitored for increases in INR and prothrombin time. Although in normal subjects no interaction with theophylline or propranolol was found, there have been clinical reports of interaction with other drugs metabolized via the cytochrome P-450 system (eg, cyclosporine, disulfiram, benzodiazepines). Patients should be monitored to determine if it is necessary to adjust the dosage of these drugs when taken concomitantly with ZEGERID.
Because of its profound and long-lasting inhibition of gastric acid secretion, it is theoretically possible that omeprazole may interfere with absorption of drugs where gastric pH is an important determinant of their bioavailability (eg, ketoconazole, ampicillin esters, and iron salts). In the clinical efficacy trials antacids were used concomitantly with the administration of omeprazole.
Concomitant administration of omeprazole and atazanavir has been reported to reduce the plasma levels of atazanavir.
Concomitant administration of omeprazole and tacrolimus may increase the serum levels of tacrolimus.
Co-administration of omeprazole and clarithromycin have resulted in increases of plasma levels of omeprazole, clarithromycin, and 14-hydroxy-clarithromycin (see also CLINICAL PHARMACOLOGY, Pharmacokinetics).
Carcinogenesis, Mutagenesis, Impairment of Fertility
In two 24-month carcinogenicity studies in rats, omeprazole at daily doses of 1.7, 3.4, 13.8, 44.0 and 140.8 mg/kg/day (approximately 0.5 to 28.5 times the human dose of 40 mg/day, based on body surface area) produced gastric ECL cell carcinoids in a dose-related manner in both male and female rats; the incidence of this effect was markedly higher in female rats, which had higher blood levels of omeprazole. Gastric carcinoids seldom occur in the untreated rat. In addition, ECL cell hyperplasia was present in all treated groups of both sexes. In one of these studies, female rats were treated with 13.8 mg omeprazole/kg/day (approximately 2.8 times the human dose of 40 mg/day, based on body surface area) for one year, then followed for an additional year without the drug. No carcinoids were seen in these rats. An increased incidence of treatment-related ECL cell hyperplasia was observed at the end of one year (94% treated vs 10% controls). By the second year the difference between treated and control rats was much smaller (46% vs 26%) but still showed more hyperplasia in the treated group. Gastric adenocarcinoma was seen in one rat (2%). No similar tumor was seen in male or female rats treated for two years. For this strain of rat no similar tumor has been noted historically, but a finding involving only one tumor is difficult to interpret. In a 52-week toxicity study in Sprague-Dawley rats, brain astrocytomas were found in a small number of males that received omeprazole at dose levels of 0.4, 2, and 16 mg/kg/day (about 0.1 to 3.3 times the human dose of 40 mg/day, based on body surface area). No astrocytomas were observed in female rats in this study. In a 2-year carcinogenicity study in Sprague-Dawley rats, no astrocytomas were found in males and females at the high dose of 140.8 mg/kg/day (about 28.5 times the human dose of 40 mg/day, based on body surface area). A 78-week mouse carcinogenicity study of omeprazole did not show increased tumor occurrence, but the study was not conclusive. A 26-week p53 (+/-) transgenic mouse carcinogenicity study was not positive. Omeprazole was positive for clastogenic effects in an in vitro human lymphocyte chromosomal aberration assay, in one of two in vivo mouse micronucleus tests, and in an in vivo bone marrow cell chromosomal aberration assay. Omeprazole was negative in the in vitro Ames Test, an in vitro mouse lymphoma cell forward mutation assay and an in vivo rat liver DNA damage assay.
Omeprazole at oral doses up to 138 mg/kg/day (about 28 times the human dose of 40 mg/day, based on body surface area) was found to have no effect on the fertility and general reproductive performance in rats.
Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies on the use of omeprazole in pregnant women. The vast majority of reported experience with omeprazole during human pregnancy is first trimester exposure and the duration of use is rarely specified, e.g., intermittent vs. chronic. An expert review of published data on experiences with omeprazole use during pregnancy by TERIS – the Teratogen Information System – concluded that therapeutic doses during pregnancy are unlikely to pose a substantial teratogenic risk (the quantity and quality of data were assessed as fair).1
Three epidemiological studies compared the frequency of congenital abnormalities among infants born to women who used omeprazole during pregnancy to the frequency of abnormalities among infants of women exposed to H2-receptor antagonists or other controls. A population-based prospective cohort epidemiological study from the Swedish Medical Birth Registry, covering approximately 99% of pregnancies, reported on 955 infants (824 exposed during the first trimester with 39 of these exposed beyond first trimester, and 131 exposed after the first trimester) whose mothers used omeprazole during pregnancy.2 In utero exposure to omeprazole was not associated with increased risk of any malformation (odds ratio 0.82, 95% CI 0.50-1.34), low birth weight or low Apgar score. The number of infants born with ventricular septal defects and the number of stillborn infants was slightly higher in the omeprazole exposed infants than the expected number in the normal population. The author concluded that both effects may be random.
A retrospective cohort study reported on 689 pregnant women exposed to either H2-blockers or omeprazole in the first trimester (134 exposed to omeprazole).3 The overall malformation rate was 4.4% (95% CI 3.6-5.3) and the malformation rate for first trimester exposure to omeprazole was 3.6% (95% CI 1.5-8.1). The relative risk of malformations associated with first trimester exposure to omeprazole compared with nonexposed women was 0.9 (95% CI 0.3-2.2). The study could effectively rule out a relative risk greater than 2.5 for all malformations. Rates of preterm delivery or growth retardation did not differ between the groups.
A controlled prospective observational study followed 113 women exposed to omeprazole during pregnancy (89% first trimester exposures).4 The reported rates of major congenital malformations was 4% for the omeprazole group, 2% for controls exposed to nonteratogens, and 2.8% in disease-paired controls (background incidence of major malformations 1-5%). Rates of spontaneous and elective abortions, preterm deliveries, gestational age at delivery, and mean birth weight did not differ between the groups. The sample size in this study has 80% power to detect a 5-fold increase in the rate of major malformation.
Several studies have reported no apparent adverse short term effects on the infant when single dose oral or intravenous omeprazole was administered to over 200 pregnant women as premedication for cesarean section under general anesthesia.
Teratology studies conducted in pregnant rats at doses up to 138 mg/kg/day (about 28 times the human dose of 40 mg/day, based on body surface area) and in pregnant rabbits at doses up to 69 mg/kg/day (about 28 times the human dose of 40 mg/day, based on body surface area) did not disclose any evidence for a teratogenic potential of omeprazole.
In rabbits, omeprazole in a dose range of 6.9 to 69 mg/kg/day (about 2.8 to 28 times the human dose of 40 mg/day, based on body surface area) produced dose-related increases in embryo-lethality, fetal resorptions and pregnancy disruptions. In rats, dose-related embryo/fetal toxicity and postnatal developmental toxicity were observed in offspring resulting from parents treated with omeprazole at 13.8 to 138 mg/kg/day (about 2.8 to 28 times the human dose of 40 mg/day, based on body surface area).
Chronic use of sodium bicarbonate may lead to systemic alkalosis and increased sodium intake can produce edema and weight increase.
Hypermagnesemia has been reported in infants whose mothers were using magnesium-containing antacid products chronically in high doses.
There are no adequate and well-controlled studies in pregnant women. Because animal studies and studies in humans cannot rule out the possibility of harm, omeprazole should be used during pregnancy only if the potential benefit to pregnant women justifies the potential risk to the fetus.
Nursing Mothers
Omeprazole concentrations have been measured in breast milk of a woman following oral administration of 20 mg. The peak concentration of omeprazole in breast milk was less than 7% of the peak serum concentration. The concentration will correspond to 0.004 mg of omeprazole in 200 mL of milk. Because omeprazole is excreted in human milk, because of the potential for serious adverse reactions in nursing infants from omeprazole, and because of the potential for tumorigenicity shown for omeprazole in rat carcinogenicity studies, a decision should be taken to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. In addition, sodium bicarbonate should be used with caution in nursing mothers.
Pediatric Use
Clinical studies have been conducted evaluating delayed-release omeprazole in pediatric patients. There are no adequate and well-controlled studies in pediatric patients with ZEGERID.
Geriatric Use
Omeprazole was administered to over 2000 elderly individuals (≥ 65 years of age) in clinical trials in the U.S. and Europe. There were no differences in safety and effectiveness between the elderly and younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Pharmacokinetic studies with buffered omeprazole have shown the elimination rate was somewhat decreased in the elderly and bioavailability was increased. The plasma clearance of omeprazole was 250 mL/min (about half that of young subjects). The plasma half-life averaged one hour, about the same as that in nonelderly, healthy subjects taking ZEGERID. However, no dosage adjustment is necessary in the elderly. (See CLINICAL PHARMACOLOGY.)
-
ADVERSE REACTIONS
Omeprazole was generally well tolerated during domestic and international clinical trials in 3096 patients.
In the U.S. clinical trial population of 465 patients, the adverse experiences summarized in Table 11 were reported to occur in 1% or more of patients on therapy with omeprazole. Numbers in parentheses indicate percentages of the adverse experiences considered by investigators as possibly, probably or definitely related to the drug.
Table 11: Adverse Experiences Occurring in 1% or More of Patients on Omeprazole Therapy Omeprazole
(n = 465)Placebo
(n = 64)Ranitidine
(n = 195)Headache 6.9 (2.4) 6.3 7.7 (2.6) Diarrhea 3.0 (1.9) 3.1 (1.6) 2.1 (0.5) Abdominal Pain 2.4 (0.4) 3.1 2.1 Nausea 2.2 (0.9) 3.1 4.1 (0.5) URI 1.9 1.6 2.6 Dizziness 1.5 (0.6) 0.0 2.6 (1.0) Vomiting 1.5 (0.4) 4.7 1.5 (0.5) Rash 1.5 (1.1) 0.0 0.0 Constipation 1.1 (0.9) 0.0 0.0 Cough 1.1 0.0 1.5 Asthenia 1.1 (0.2) 1.6 (1.6) 1.5 (1.0) Back Pain 1.1 0.0 0.5 Table 12 summarizes the adverse reactions that occurred in 1% or more of omeprazole-treated patients from international double-blind, and open-label clinical trials in which 2,631 patients and subjects received omeprazole.
Table 12: Incidence of Adverse Experiences ≥ 1%; Causal Relationship not Assessed Omeprazole
(n = 2631)Placebo
(n = 120)Body as a Whole, site unspecified Abdominal pain 5.2 3.3 Asthenia 1.3 0.8 Digestive System Constipation 1.5 0.8 Diarrhea 3.7 2.5 Flatulence 2.7 5.8 Nausea 4.0 6.7 Vomiting 3.2 10.0 Acid regurgitation 1.9 3.3 Nervous System/Psychiatric Headache 2.9 2.5 Additional adverse experiences occurring in < 1% of patients or subjects in domestic and/or international trials conducted with omeprazole, or occurring since the drug was marketed, are shown below within each body system. In many instances, the relationship to omeprazole was unclear.
Body As a Whole
Allergic reactions, including, rarely, anaphylaxis (see also Skin below), fever, pain, fatigue, malaise, abdominal swelling.
Cardiovascular
Chest pain or angina, tachycardia, bradycardia, palpitation, elevated blood pressure, and peripheral edema.
Gastrointestinal
Pancreatitis (some fatal), anorexia, irritable colon, flatulence, fecal discoloration, esophageal candidiasis, mucosal atrophy of the tongue, dry mouth, stomatitis. During treatment with omeprazole, gastric fundic gland polyps have been noted rarely. These polyps are benign and appear to be reversible when treatment is discontinued.
Gastroduodenal carcinoids have been reported in patients with Zollinger-Ellison syndrome on long-term treatment with omeprazole. This finding is believed to be a manifestation of the underlying condition, which is known to be associated with such tumors.
Hepatic
Mild and, rarely, marked elevations of liver function tests [ALT (SGPT), AST (SGOT), γ-glutamyl transpeptidase, alkaline phosphatase, and bilirubin (jaundice)]. In rare instances, overt liver disease has occurred, including hepatocellular, cholestatic, or mixed hepatitis, liver necrosis (some fatal), hepatic failure (some fatal), and hepatic encephalopathy.
Metabolic/Nutritional
Hyponatremia, hypoglycemia, and weight gain.
Musculoskeletal
Muscle cramps, myalgia, muscle weakness, joint pain, and leg pain.
Nervous System/Psychiatric
Psychic disturbances including depression, agitation, aggression, hallucinations, confusion, insomnia, nervousness, tremors, apathy, somnolence, anxiety, dream abnormalities; vertigo; paresthesia; and hemifacial dysesthesia.
Respiratory
Epistaxis, pharyngeal pain.
Skin
Rash and rarely, cases of severe generalized skin reactions including toxic epidermal necrolysis (TEN; some fatal), Stevens-Johnson syndrome, and erythema multiforme (some severe); purpura and/or petechiae (some with rechallenge); skin inflammation, urticaria, angioedema, pruritus, photosensitivity, alopecia, dry skin, and hyperhidrosis.
Special Senses
Tinnitus, taste perversion.
Ocular
Blurred vision, ocular irritation, dry eye syndrome, optic atrophy, anterior ischemic optic neuropathy, optic neuritis and double vision.
Urogenital
Interstitial nephritis (some with positive rechallenge), urinary tract infection, microscopic pyuria, urinary frequency, elevated serum creatinine, proteinuria, hematuria, glycosuria, testicular pain, and gynecomastia.
Hematologic
Rare instances of pancytopenia, agranulocytosis (some fatal), thrombocytopenia, neutropenia, leukopenia, anemia, leucocytosis, and hemolytic anemia have been reported.
The incidence of clinical adverse experiences in patients greater than 65 years of age was similar to that in patients 65 years of age or less.
Additional adverse reactions that could be caused by sodium bicarbonate include metabolic alkalosis, seizures, and tetany.
The use of magnesium hydroxide is associated with diarrhea, abdominal cramping, chalky taste, diuresis, dehydration, nausea, and vomiting.
-
OVERDOSAGE
Reports have been received of overdosage with omeprazole in humans. Doses ranged up to 2400 mg (120 times the usual recommended clinical dose). Manifestations were variable, but included confusion, drowsiness, blurred vision, tachycardia, nausea, vomiting, diaphoresis, flushing, headache, dry mouth, and other adverse reactions similar to those seen in normal clinical experience. (See ADVERSE REACTIONS.) Symptoms were transient, and no serious clinical outcome has been reported when omeprazole was taken alone. No specific antidote for omeprazole overdosage is known. Omeprazole is extensively protein bound and is, therefore, not readily dialyzable. In the event of overdosage, treatment should be symptomatic and supportive.
As with the management of any overdose, the possibility of multiple drug ingestion should be considered. For current information on treatment of any drug overdose, a certified Regional Poison Control Center should be contacted. Telephone numbers are listed in the Physicians' Desk Reference (PDR) or local telephone book.
Single oral doses of omeprazole at 1350, 1339, and 1200 mg/kg were lethal to mice, rats, and dogs, respectively. Animals given these doses showed sedation, ptosis, tremors, convulsions, and decreased activity, body temperature, and respiratory rate and increased depth of respiration.
In addition, a sodium bicarbonate overdose may cause hypocalcemia, hypokalemia, hypernatremia, and seizures.
Similarly, a magnesium overdose may lead to hypermagnesemia. Hypermagnesemia results in a depressant effect on the central nervous system, causing anorexia and nausea, and neuromuscular system. Magnesium toxicity causes hypotension, muscle weakness, and electrographic changes.
-
DOSAGE AND ADMINISTRATION
ZEGERID with Magnesium Hydroxide (omeprazole/sodium bicarbonate/magnesium hydroxide) is available as chewable tablets in 20 mg and 40 mg strengths for adult use. Directions for use for each indication are summarized in Table 13.
Because ZEGERID chewable tablets contain magnesium hydroxide, the chewable tablets should not be substituted for other dosage forms (eg, ZEGERID Powder for Oral Suspension or ZEGERID Capsules).
Since both the 20-mg and 40-mg chewable tablets contain the same amount of sodium bicarbonate (600 mg) and magnesium hydroxide (700 mg), two 20-mg chewable tablets are not equivalent to one 40-mg chewable tablet; therefore, two 20-mg chewable tablets should not be substituted for one 40-mg chewable tablet.
ZEGERID with Magnesium Hydroxide should be taken on an empty stomach at least one hour before a meal.
Table 13: Recommended Doses of ZEGERID® with Magnesium Hydroxide by Indication for Adults 18 Years and Older Indication Recommended Dose Frequency * Most patients heal within 4 weeks. Some patients may require an additional 4 weeks of therapy.
** For additional information, see CLINICAL PHARMACOLOGY, Clinical Studies section
+ For additional information, see INDICATIONS AND USAGE section
Short-Term Treatment of Active
Duodenal Ulcer20 mg Once daily for 4 weeks*,+ Benign Gastric Ulcer 40 mg Once daily for 4-8 weeks **,+ Gastroesophageal Reflux Disease (GERD)
Symptomatic GERD
(with no esophageal erosions)20 mg Once daily for up to 4 weeks+
Erosive Esophagitis 20 mg Once daily for 4-8 weeks+ Maintenance of Healing of Erosive Esophagitis 20 mg Once daily** -
HOW SUPPLIED
ZEGERID with Magnesium Hydroxide 20-mg Chewable Tablets: Each pink, 18 mm in diameter, round tablet, inscribed with the number “2031” on one side and the Santarus logo on the other side, contains 20 mg omeprazole and 600 mg sodium bicarbonate plus 700 mg magnesium hydroxide.
NDC: 68012-152-30 Bottles of 30 chewable tablets
ZEGERID with Magnesium Hydroxide 40-mg Chewable Tablets: Each pink, 18 mm in diameter, round tablet, inscribed with the number “4031” on one side and the Santarus logo on the other side, contains 40 mg omeprazole and 600 mg sodium bicarbonate plus 700 mg magnesium hydroxide.
NDC: 68012-154-30 Bottles of 30 chewable tablets
-
REFERENCES
- Friedman JM and Polifka JE. Omeprazole. In: Teratogenic Effects of Drugs. A Resource for Clinicians (TERIS). 2nd ed. Baltimore, MD: The Johns Hopkins University Press 2000; p. 516.
- Kallen BAJ. Use of omeprazole during pregnancy – no hazard demonstrated in 955 infants exposed during pregnancy. Eur Obstet Gynecol Reprod Biol 2001; 96(1):63-8.
- Ruigómez A, Rodriguez LUG, Cattaruzzi C, et al. Use of cimetidine, omeprazole, and ranitidine in pregnant women and pregnancy outcomes. Am J Epidemiol 1999; 150:476-81.
- Lalkin A, Loebstein, Addis A, et al. The safety of omeprazole during pregnancy: a multicenter prospective controlled study. Am J Obstet Gynecol 1998; 179:727-30.
SANTARUS, INC.®
ZEGERID® with Magnesium Hydroxide Chewable Tablets are manufactured for Santarus, Inc., San Diego, CA 92130
by: Patheon Pharmaceuticals, Inc. Cincinnati, OH 45237
For more information call 1-888-778-0887
Revised: March 2006
ZEGERID® is a registered trademark of Santarus, Inc.
© 2006 Santarus, Inc.
-
SUPPLEMENTAL PATIENT MATERIAL
Information for Patients
ZEGERID with Magnesium Hydroxide should be taken on an empty stomach at least one hour prior to a meal. ZEGERID with Magnesium Hydroxide chewable tablets are available in 40 mg and 20 mg dosage strengths of omeprazole with 600 mg sodium bicarbonate plus 700 mg magnesium hydroxide per tablet.
Directions for Use: DO NOT SWALLOW WHOLE AND DO NOT SUBSTITUTE FOR OTHER ZEGERID DOSAGE FORMS. Chew the tablet and swallow with water. DO NOT USE OTHER LIQUIDS.
-
INGREDIENTS AND APPEARANCE
ZEGERID WITH MAGNESIUM HYDROXIDE
omeprazole, sodium bicarbonate and magnesium hydroxide tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68012-152 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength omeprazole (UNII: KG60484QX9) (omeprazole - UNII:KG60484QX9) 20 mg sodium bicarbonate (UNII: 8MDF5V39QO) (sodium bicarbonate - UNII:8MDF5V39QO) 600 mg magnesium hydroxide (UNII: NBZ3QY004S) (magnesium hydroxide - UNII:NBZ3QY004S) 700 mg Inactive Ingredients Ingredient Name Strength hydroxypropyl cellulose () croscarmellose sodium () xylitol () sucralose () flavoring () magnesium stearate (UNII: 70097M6I30) FD&C #40 Aluminum Lake () Product Characteristics Color PINK (PINK) Score no score Shape ROUND (ROUND) Size 18mm Flavor Imprint Code 2031 Contains Coating false Symbol true Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68012-152-30 30 in 1 BOTTLE, PLASTIC ZEGERID WITH MAGNESIUM HYDROXIDE
omeprazole, sodium bicarbonate and magnesium hydroxide tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68012-154 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength omeprazole (UNII: KG60484QX9) (omeprazole - UNII:KG60484QX9) 40 mg sodium bicarbonate (UNII: 8MDF5V39QO) (sodium bicarbonate - UNII:8MDF5V39QO) 600 mg magnesium hydroxide (UNII: NBZ3QY004S) (magnesium hydroxide - UNII:NBZ3QY004S) 700 mg Inactive Ingredients Ingredient Name Strength hydroxypropyl cellulose () croscarmellose sodium () xylitol () sucralose () flavoring () magnesium stearate (UNII: 70097M6I30) FD&C #40 Aluminum Lake () Product Characteristics Color PINK (PINK) Score no score Shape ROUND (ROUND) Size 18mm Flavor Imprint Code 4031 Contains Coating false Symbol true Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68012-154-30 30 in 1 BOTTLE, PLASTIC Labeler - Santarus, Inc.
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.