70% dextrose injection usp
Dextrose by
Drug Labeling and Warnings
Dextrose by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Company, Baxter Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DEXTROSE- dextrose monohydrate injection, solution
Baxter Healthcare Company
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
70% dextrose injection usp
Health Care Professional Letter
Reporting Adverse Events or Product Quality Issues
To report adverse events associated with these imported products, please call Baxter at 1-866-888-2472, or fax: 1-800-759-1801. Adverse events or quality problems experienced with the use of these imported products may also be reported to the FDA’s MedWatch Adverse Event Reporting program either online, or by regular mail or by fax:
- Complete and submit the report Online: https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program
- Regular mail or Fax: Download form https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
To report product quality issues associated with these imported products, please contact Baxter Product Surveilance through Baxter Product Feedback Portal https://productfeedback.baxter.com/
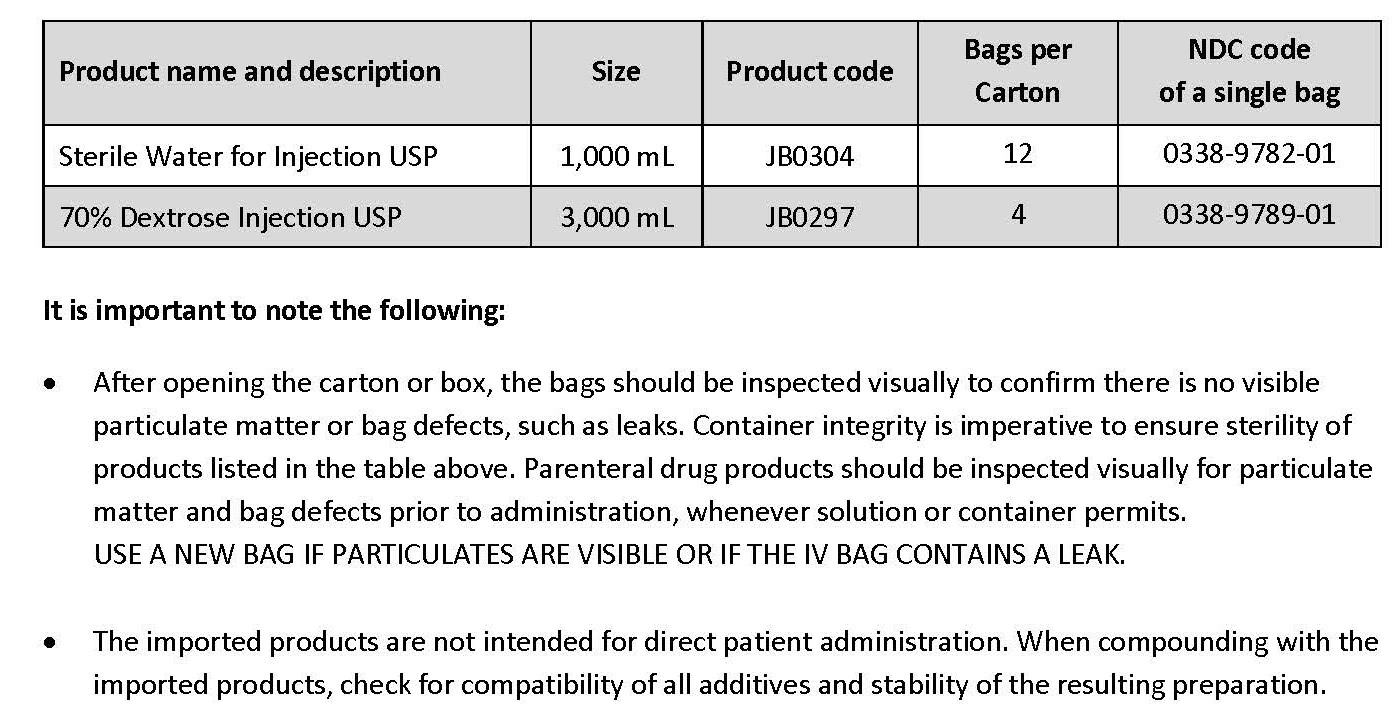
Please refer to the FDA-approved prescribing information for each drug product listed below:
- Sterile Water for Injection USP (click https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018632s051lbl.pdf)
- 70% Dextrose Injection USP (click https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020047s021lbl.pdf)
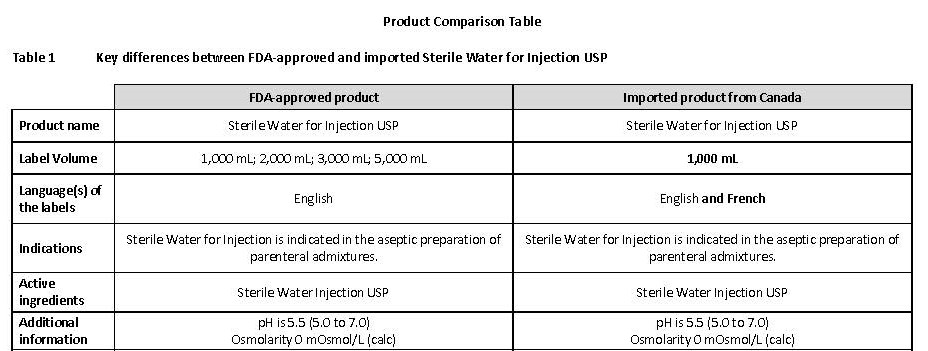
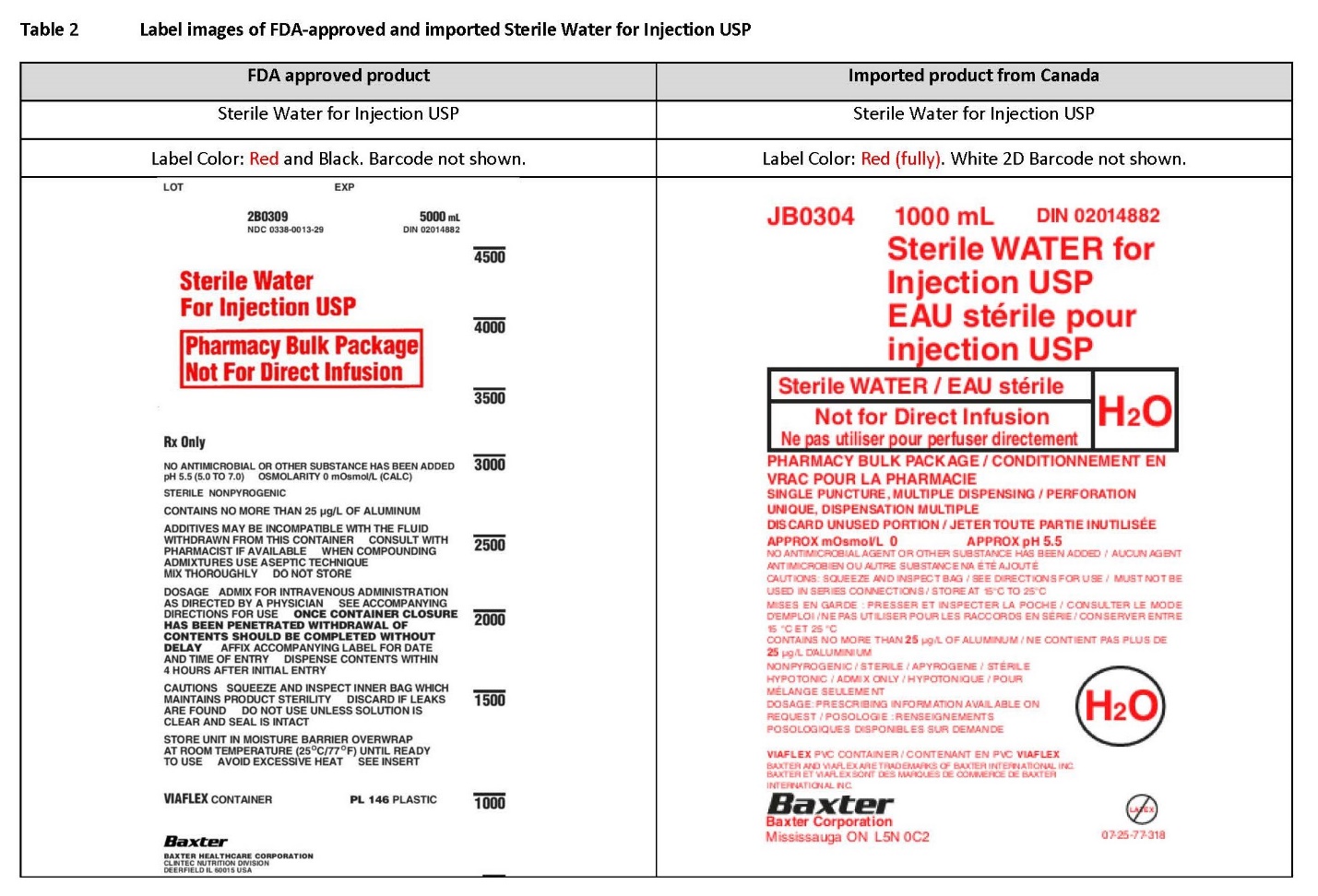
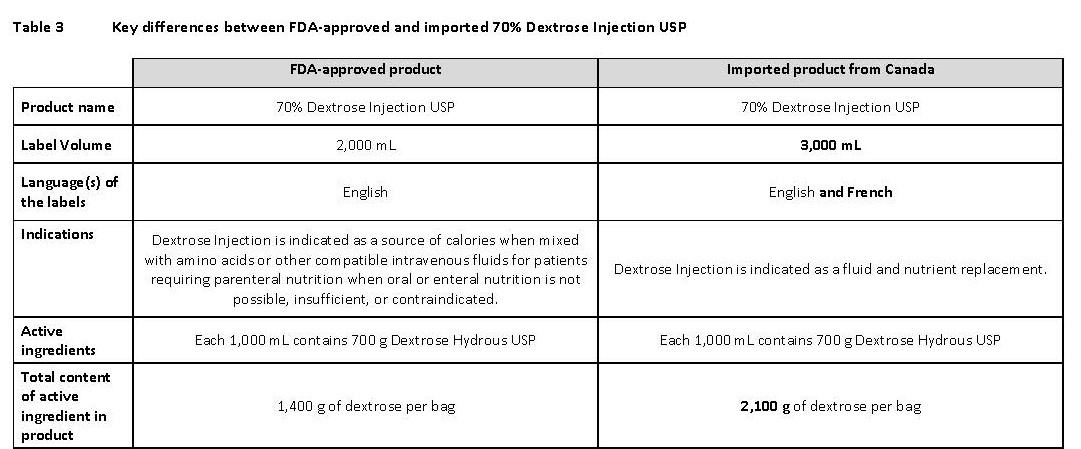
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Container Label
JB0297 3000 mL DIN 02014874
70% Dextrose Injection USP
Dextrose à 70% USP, Injectable
DEXTROSE 70%
2D Barcode
(01)00809080000654
Pharmacy Use Only / Dilute
Before Infusing
Pour Usage Par La Pharma-
cie Seulement / Diluer Avant
La Perfusion
Not for Direct Infusion /
Ne pa utilier pour perfuser directement
HYPERTONIC / HYPERTONIQUE
CONTAINS NO MORE THAN 25 µg/l OF ALUMINUM /
NE CONTENT PAS PLUS DE 25 µg/l D’ALUMINIUM
APPROX mOsmol/L – 3530 APPROX pH 4.0
INTRAVENOUS FLUID AND NUTRIENT REPLENISHMENT /
RECHARGE LIQUIDIENNE ET NUTRIMENT PAR
INJECTION INTRAVEINEUSE
PER 100 mL DEXTROSE HYDROUS USP – 70 g / WATER
FOR INJECTION USP qs
PAR 100 mL DEXTROSE HYDRATE USP – 70 g / EAU
POUR INJETION USP qs
COLOUR VARIATION FROM LIGHT YELLOW TO AMBER IS
NORMAL AND DOES NOT ALTER EFFICACY / IL EST NOR-
MAL QUE LA COULEUR VARIE D’UN JAUNE PALE A UN
JAUNE AMBRE ET CELA N’AFFECTE PAS L’EFFICACITE
AFFIX ACCOMPANYING LABEL FOR DATE AND TIME OF
ENTRY / APPOSER UNE ETIQUETTE ET INSCRIRE LA
DATE ET L’HEURE DU PRELEVEMENT INITIAL / DISCARD
UNUSED CONTENTS WITHIN 4 HOURS OF INITIAL ENTRY
/ JETER LE CONTENANT 4 HEURES APRES LE PREMIER
PRELEVEMENT
CAUTIONS SQUEEZE AND INSPECT BAG / SEE DIREC-
TIONS FOR USE / STORE AT 15°C TO 25°C
ATTENTIONS PRESSER ET INSPECTER LE SAC / VOIR
MODE D’EMPLOI / GARDER ENTIRE 15°C ET 25°C
NONPYROGENIC / STERILE / APYROGENE / STERILE
PRESCRIBING INFORMATION AVAILABLE ON REQUEST / INFORMA-
TION POSOLOGIQUE DISPONIBLE SUR DEMANDE
VIAFLEX PVC CONTAINER/CONTENANT DE PVC
BAXTER AND VIAFLEX ARE TRADEMARKS OF BAXTER INTERNATIONAL INC
BAXTER ET VIAFLEX SONT DE MARQUES DE COMMERCE DE BAXTER
INTERNATIONAL INC
Baxter Logo
BAXTER CORPORATION
Mississauga ON L5N 0C2
70%
No Latex Label
88-70-20-462
2100
1800
1500
1200
900
600
300
JB-02-97
4 – 3000 mL units
Store at 15°C to 25°C
70% Dextrose Injection USP
Lot:WWWWWWWWW EXP: 2099 99
2D Barcode
(01)20809080000658
Pharmacy Bulk
| DEXTROSE
dextrose monohydrate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Baxter Healthcare Company (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Corporation | 205087968 | ANALYSIS(0338-9789) , LABEL(0338-9789) , MANUFACTURE(0338-9789) , STERILIZE(0338-9789) , PACK(0338-9789) | |