DOCOSANOL by Cosette Pharmaceuticals, Inc. / Alembic Pharmaceuticals Limited DOCOSANOL cream
DOCOSANOL by
Drug Labeling and Warnings
DOCOSANOL by is a Otc medication manufactured, distributed, or labeled by Cosette Pharmaceuticals, Inc., Alembic Pharmaceuticals Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Uses
-
Warnings
This product may cause a severe allergic reaction. Symptoms may include:
- hives
- facial swelling
- wheezing/difficulty breathing
- shock
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
For external use only
Do not use
- if you are allergic to any ingredient in this product
- apply only to the affected areas
- do not use in or near the eyes
- avoid applying directly inside your mouth
- do not share this product with anyone. This may spread the infection.
- your cold sore gets worse or the cold sore is not healed within 10 days
- Keep out of reach of children.
-
Directions
- adults and children 12 years or over:
wash hands before and after applying cream
apply to affected area on face or lips at the first sign of cold sore/fever blister (tingle)
early treatment ensures the best results
rub in gently but completely
use 5 times a day until healed
- children under 12 years: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
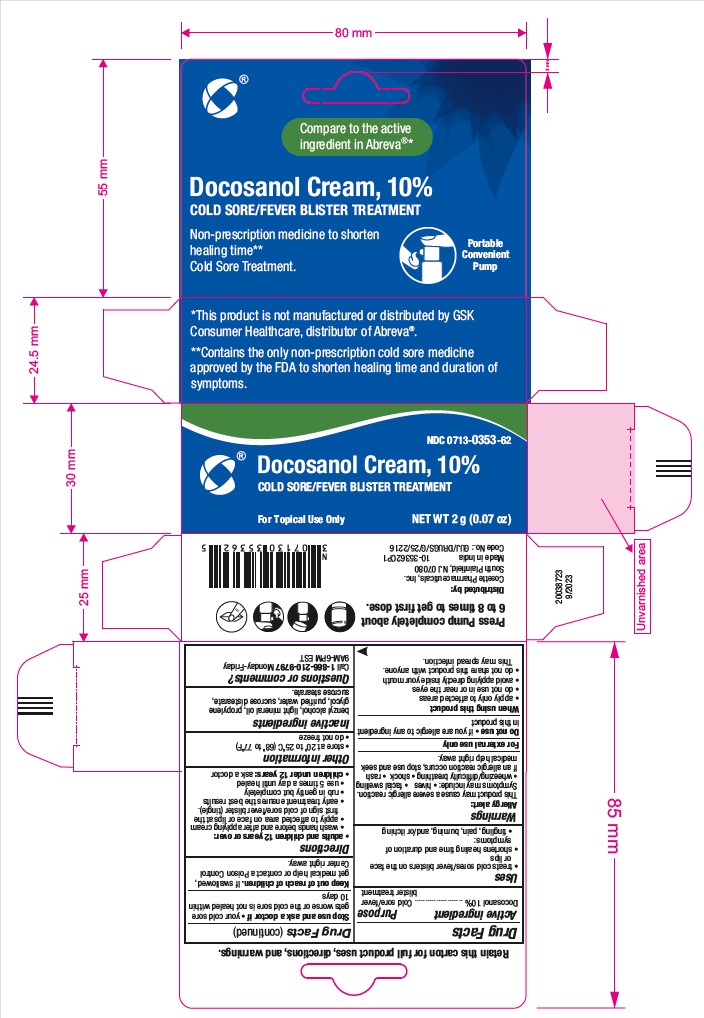
Principal Display Panel
NDC: 0713-0353-02
Docosanol Cream, 10%
Compare to the active ingredient in Abreva®**
Cold Sore/Fever Blister Treatment
Non-prescription Medicine to Shorten Healing Time*
Cold Sore Treatment
For Topical Use Only
NET WT 2g (0.07 oz)
**This product is not manufactured or distributed by GSK Consumer Healthcare, distributor of Abreva®.
*Contains the only non-prescription cold sore medicine approved by the FDA to shorten healing time and duration of symptoms.
RETAIN THIS INSERT FOR FULL PRODUCT USES, DIRECTIONS AND WARNINGS
Distributed by:
Cosette Pharmaceuticals, Inc.
South Plainfield, NJ 07080
Made in India.
Code No.: GUJ/DRUGS/G/25/2216

2
NDC: 0713-0353-62
Docosanol Cream, 10%
Compare to the active ingredient in Abreva®*
Cold Sore/Fever Blister Treatment
Non-prescription Medicine to Shorten Healing Time**
Cold Sore Treatment
For Topical Use Only
NET WT 2g (0.07 oz)
*This product is not manufactured or distributed by GSK Consumer Healthcare, distributor of Abreva®.
**Contains the only non-prescription cold sore medicine approved by the FDA to shorten healing time and duration of symptoms.
RETAIN THIS INSERT FOR FULL PRODUCT USES, DIRECTIONS AND WARNINGS
Distributed by:
Cosette Pharmaceuticals, Inc.
South Plainfield, NJ 07080
Made in India.
Code No.: GUJ/DRUGS/G/25/2216
4
-
INGREDIENTS AND APPEARANCE
DOCOSANOL
docosanol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0713-0353 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCOSANOL (UNII: 9G1OE216XY) (DOCOSANOL - UNII:9G1OE216XY) DOCOSANOL 100 mg in 1 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) LIGHT MINERAL OIL (UNII: N6K5787QVP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SUCROSE DISTEARATE (UNII: 33X4X4B90S) SUCROSE STEARATE (UNII: 274KW0O50M) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0713-0353-02 1 in 1 CARTON 01/04/2024 1 2 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 0713-0353-62 1 in 1 CARTON 01/04/2024 2 2 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215839 01/04/2024 Labeler - Cosette Pharmaceuticals, Inc. (116918230) Registrant - Alembic Pharmaceuticals Limited (650574663) Establishment Name Address ID/FEI Business Operations Alembic Pharmaceuticals Limited 871411532 MANUFACTURE(0713-0353) , ANALYSIS(0713-0353)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.