QVAR REDIHALER- beclomethasone dipropionate hfa aerosol, metered
QVAR REDIHALER by
Drug Labeling and Warnings
QVAR REDIHALER by is a Prescription medication manufactured, distributed, or labeled by A-S Medication Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use QVAR REDIHALER safely and effectively. See full prescribing information for QVAR REDIHALER.
QVAR® REDIHALER™ (beclomethasone dipropionate HFA) inhalation aerosol, for oral inhalation use
Initial U.S. Approval: 1976INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
For oral inhalation only. (2.1)
- Starting dosage is based on prior asthma therapy and disease severity. (2.2)
- Treatment of asthma in patients 4 to 11 years of age: 40 or 80 mcg twice daily. (2.2)
- Treatment of asthma in patients 12 years of age and older: 40 mcg, 80 mcg, 160 mcg, or 320 mcg twice daily (2.2)
- Discard QVAR REDIHALER inhaler when the dose counter displays 0 or after the expiration date on the product, whichever comes first. (2.1)
- Do not use a spacer or volume holding chamber (2.1)
DOSAGE FORMS AND STRENGTHS
- Breath-actuated inhalation aerosol: 40 or 80 mcg per actuation. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Localized infections: Candida albicans infection of the mouth and throat may occur. Monitor patients periodically for signs of adverse effects on the oral cavity. Advise patients to rinse the mouth with water without swallowing after inhalation to help reduce the risk. (5.1)
- Deterioration of asthma and acute episodes: Do not use QVAR REDIHALER for relief of acute symptoms. Patients require immediate re-evaluation during rapidly deteriorating asthma. (5.2)
- Transferring patients from systemic corticosteroids: Risk of impaired adrenal function when transferring from oral steroids. Taper patients slowly from systemic corticosteroids if transferring to QVAR REDIHALER. (5.3)
- Immunosuppression: Potential worsening of existing tuberculosis, fungal, bacterial, viral, or parasitic infection; or ocular herpes simplex infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients. Use with caution in patients with these infections because of the potential for worsening of these infections. (5.4)
- Paradoxical bronchospasm: Bronchospasm, with an immediate increase in wheezing, may occur after dosing. Treat bronchospasm immediately with inhaled, short acting bronchodilator and discontinue QVAR REDIHALER. (5.5)
- Hypersensitivity reactions: Hypersensitivity reactions, such as urticaria, angioedema, rash, and bronchospasm may occur. Discontinue QVAR REDIHALER if such reactions occur. (5.6)
- Hypercorticism and adrenal suppression: May occur with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue QVAR REDIHALER slowly. (5.7)
- Effects on growth: Monitor growth of pediatric patients. (5.8)
- Decreases in bone mineral density: Monitor patients with major risk factors for decreased bone mineral content. (5.9)
- Eye Disorders: Monitor patients with change in vision or with a history of increased intraocular pressure, blurred vision, glaucoma, and/or cataracts closely. (5.10)
ADVERSE REACTIONS
Most common adverse reactions (incidence >3% and > placebo) include oral candidiasis, upper respiratory tract infection, nasopharyngitis, allergic rhinitis, oropharyngeal pain and sinusitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals at 1-888-483-8279 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Administration Information
2.2 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Status Asthmaticus
4.2 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Local Effects
5.2 Deterioration of Asthma and Acute Episodes
5.3 Transferring Patients from Systemic Corticosteroid Therapy
5.4 Immunosuppression
5.5 Paradoxical Bronchospasm
5.6 Immediate Hypersensitivity Reactions
5.7 Hypercorticism and Adrenal Suppression
5.8 Effects on Growth
5.9 Reduction in Bone Mineral Density
5.10 Eye Disorders
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Trials in the Maintenance Treatment of Asthma
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Administration Information
Administer QVAR REDIHALER by the orally inhaled route in patients 4 years of age and older. After inhalation, the patient should rinse his/her mouth with water without swallowing to help reduce the risk of oropharyngeal candidiasis.
Patients should be instructed on the proper use of their inhaler.
Consistent dose delivery is achieved, whether using the 40‑ or 80‑mcg strengths, due to proportionality of the 2 products (i.e., 2 actuations of 40‑mcg strength should provide a dose comparable to 1 actuation of the 80‑mcg strength).
Priming: QVAR REDIHALER does not require priming.
Shaking the inhaler prior to use is not necessary. Do not shake the inhaler with the cap open to avoid possible actuation of the device.
Do not use QVAR REDIHALER with a spacer or volume holding chamber.
Cleaning:
- Keep the inhaler clean and dry at all times. Never wash or put any part of the inhaler in water.
- Routine maintenance is not required. If the mouthpiece needs cleaning, gently wipe the mouthpiece with a dry cloth or tissue as needed.
Dose Counter: QVAR REDIHALER has a dose counter attached to the actuator. When the patient receives the inhaler, the number 120 will be displayed. The dose counter will count down each time a spray is released. When the dose counter reaches 20, the color of the numbers will change to red to remind the patient to contact their pharmacist for a refill of medication or consult their physician for a prescription refill. When the dose counter reaches 0, the background will change to solid red. Discard QVAR REDIHALER inhaler when the dose counter displays 0 or after the expiration date on the product, whichever comes first [see Patient Counseling Information (17)].
2.2 Recommended Dosage
Adults and Adolescents 12 years of age and older
The starting dosage is based on previous asthma therapy and disease severity, including consideration of the patients’ current control of asthma symptoms and risk of future exacerbation. The recommended starting dosage for patients 12 years of age and older who are not on an inhaled corticosteroid is 40 to 80 mcg twice daily, approximately 12 hours apart. For patients switching to QVAR REDIHALER from another inhaled corticosteroid product, select the appropriate starting dosage strength of QVAR REDIHALER based on the strength of the previous inhaled corticosteroid product and disease severity: 40, 80, 160 or 320 mcg twice daily. For patients who do not respond adequately to the initial dosage after 2 weeks of therapy, increasing the dosage may provide additional asthma control. The maximum recommended dosage for patients 12 years of age and older is 320 mcg twice daily.
Pediatric Patients 4 to 11 years
The starting dosage is based on previous asthma therapy and disease severity, including consideration of the patients’ current control of asthma symptoms and risk of future exacerbation. The recommended starting dosage for patients aged 4 to 11 years of age is 40 mcg twice daily, approximately 12 hours apart. For patients who do not respond adequately to QVAR REDIHALER 40 mcg after 2 weeks of therapy, increasing the dosage to QVAR REDIHALER 80 mcg twice daily may provide additional asthma control. The maximum recommended dosage for patients 4 to 11 years of age is 80 mcg twice daily.
General Dosing Recommendations
The onset and degree of symptom relief will vary in individual patients. Improvement in asthma symptoms can occur within 24 hours of the beginning of treatment and should be expected within the first or second week, but maximum benefit should not be expected until 3 to 4 weeks of therapy. Improvement in pulmonary function is usually apparent within 1 to 4 weeks after the start of therapy.
If a dosage regimen of QVAR REDIHALER fails to provide adequate control of asthma, the therapeutic regimen should be re-evaluated and additional therapeutic options (e.g., replacing the current strength of QVAR REDIHALER with a higher strength, or adding additional controller therapies) should be considered.
As with any inhaled corticosteroid, physicians are advised to titrate the dose of QVAR REDIHALER downward over time to the lowest level that maintains proper asthma control. This is particularly important in children since a controlled study has shown that beclomethasone dipropionate has the potential to affect growth in children.
The maximum number of inhalations should not exceed 8 per day.
-
3 DOSAGE FORMS AND STRENGTHS
Inhalation Aerosol. QVAR REDIHALER is a pressurized, breath‑actuated, metered-dose aerosol with a dose counter intended for oral inhalation containing beclomethasone dipropionate in the following 2 strengths:
- QVAR REDIHALER 40 mcg is supplied in an aluminum canister contained within a beige plastic actuator with a dose counter and a hinged white cap. Each breath‑induced actuation delivers 50 mcg from the valve and 40 mcg from the actuator. QVAR REDIHALER 40 mcg is available as a 120‑inhalation/10.6‑g canister.
- QVAR REDIHALER 80 mcg is supplied in an aluminum canister contained within a maroon plastic actuator with a dose counter and a hinged white cap. Each breath‑induced actuation delivers 100 mcg of beclomethasone dipropionate from the valve and 80 mcg from the actuator. QVAR REDIHALER 80 mcg is available as a 120‑inhalation/10.6‑g canister.
-
4 CONTRAINDICATIONS
4.1 Status Asthmaticus
QVAR REDIHALER is contraindicated in the primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required [see Warnings and Precautions (5.2)].
4.2 Hypersensitivity
QVAR REDIHALER is contraindicated in patients with known hypersensitivity to beclomethasone dipropionate or any of the ingredients in QVAR REDIHALER [see Warnings and Precautions (5.6)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Local Effects
Localized infections with Candida albicans have occurred in the mouth and pharynx in some patients receiving QVAR REDIHALER. If oropharyngeal candidiasis develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while still continuing with QVAR REDIHALER therapy, but at times therapy with QVAR REDIHALER may need to be temporarily interrupted under close medical supervision. After inhalation, the patient should rinse his/her mouth with water without swallowing to help reduce the risk of oropharyngeal candidiasis.
5.2 Deterioration of Asthma and Acute Episodes
QVAR REDIHALER is not indicated for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. An inhaled, short‑acting beta2‑agonist, not QVAR REDIHALER, should be used to relieve acute symptoms such as shortness of breath. Instruct patients to contact their physician immediately if episodes of asthma that are not responsive to bronchodilators occur during the course of treatment with QVAR REDIHALER. During such episodes, patients may require therapy with oral corticosteroids.
5.3 Transferring Patients from Systemic Corticosteroid Therapy
Particular care is needed in patients who are transferred from systemically active corticosteroids to QVAR REDIHALER because deaths due to adrenal insufficiency have occurred in asthmatic patients during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic‑pituitary‑adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infections (particularly gastroenteritis) or other conditions with severe electrolyte loss. Although QVAR REDIHALER may provide control of asthmatic symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of glucocorticoid systemically and does NOT provide the mineralocorticoid that is necessary for coping with these emergencies.
During periods of stress or a severe asthmatic attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their physician for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic steroids during periods of stress or a severe asthma attack.
Patients requiring oral or other systemic corticosteroids should be weaned slowly from oral or other systemic corticosteroid use after transferring to QVAR REDIHALER. Lung function (FEV1 or PEF), beta‑agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral or other systemic corticosteroids. In addition to monitoring asthma signs and symptoms, patients should be observed for signs and symptoms of adrenal insufficiency such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Transfer of patients from systemic corticosteroid therapy to QVAR REDIHALER may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy, e.g., rhinitis, conjunctivitis, eczema, arthritis, and eosinophilic conditions.
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal, e.g., joint and/or muscular pain, lassitude, and depression, despite maintenance or even improvement of respiratory function.
5.4 Immunosuppression
Persons who are on drugs which suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in non-immune patients on corticosteroids. In such patients who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. It is not known how the dose, route and duration of corticosteroid administration affect the risk of developing a disseminated infection, and nor is the contribution of the underlying disease and/or prior corticosteroid treatment known. If exposed to chickenpox, prophylaxis with varicella-zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
Inhaled corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract; untreated systemic fungal, bacterial, parasitic or viral infections; or ocular herpes simplex.
5.5 Paradoxical Bronchospasm
Inhaled corticosteroids may produce inhalation‑induced bronchospasm with an immediate increase in wheezing after dosing that may be life-threatening. If inhalation induced bronchospasm occurs following dosing with QVAR REDIHALER, it should be treated immediately with an inhaled, short‑acting bronchodilator. Treatment with QVAR REDIHALER should be discontinued and alternate therapy instituted.
5.6 Immediate Hypersensitivity Reactions
Hypersensitivity reactions, such as urticaria, angioedema, rash, and bronchospasm, may occur after administration of QVAR REDIHALER. Discontinue QVAR REDIHALER if such reactions occur [see Contraindications (4.2)].
5.7 Hypercorticism and Adrenal Suppression
QVAR REDIHALER will often help control asthma symptoms with less suppression of HPA function than therapeutically equivalent oral doses of prednisone. Since beclomethasone dipropionate is absorbed into the circulation and can be systemically active at higher doses, the beneficial effects of QVAR REDIHALER in minimizing HPA dysfunction may be expected only when recommended dosages are not exceeded and individual patients are titrated to the lowest effective dose.
Because of the possibility of systemic absorption of inhaled corticosteroids, patients treated with QVAR REDIHALER should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients, particularly when beclomethasone dipropionate is administered at higher than recommended doses over prolonged periods of time. If such effects occur, the dosage of QVAR REDIHALER should be reduced slowly, consistent with accepted procedures for reducing systemic corticosteroids and for management of asthma symptoms.
5.8 Effects on Growth
Orally inhaled corticosteroids, including QVAR REDIHALER, may cause a reduction in growth velocity when administered to pediatric patients. Monitor the growth of pediatric patients receiving QVAR REDIHALER routinely (e.g., via stadiometry). To minimize the systemic effects of orally inhaled corticosteroids, including QVAR REDIHALER, titrate each patient’s dose to the lowest dosage that effectively controls his/her symptoms [see Use in Specific Populations(8.4)].
5.9 Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long‑term administration of products containing inhaled corticosteroids. The clinical significance of small changes in BMD with regard to long‑term outcomes, such as fracture, is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants and corticosteroids) should be monitored and treated with established standards of care.
5.10 Eye Disorders
Glaucoma, increased intraocular pressure, blurred vision and cataracts have been reported following the use of long term administration of inhaled corticosteroids. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, blurred vision, glaucoma, and/or cataracts while using QVAR REDIHALER.
-
6 ADVERSE REACTIONS
Systemic and local corticosteroid use may result in the following:
- Candida albicans infection [see Warnings and Precautions (5.1)]
- Immunosuppression [see Warnings and Precautions (5.4)]
- Hypercorticism and adrenal suppression [see Warnings and Precautions (5.7)]
- Growth effects [see Warnings and Precautions (5.8) and Use in Specific Populations (8.4)]
- Eye Disorders [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
A total of 1858 subjects participated in the QVAR REDIHALER clinical development program. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults and Adolescent Patients 12 years of Age and Older: The adverse reaction information presented in Table 1 is derived from 3 double-blind, placebo-controlled clinical trials in which 1230 patients (751 female and 479 male adults previously treated with as‑needed bronchodilators and/or inhaled corticosteroids) were treated with QVAR REDIHALER (doses of 40, 80, 160, or 320 mcg twice daily) or QVAR (beclomethasone dipropionate HFA) Inhalation Aerosol (QVAR MDI; doses of 160 or 320 mcg twice daily) or placebo. In considering these data, difference in average duration of exposure and clinical trial design should be taken into account.
Table 1 Adverse Reactions Experienced by at Least 3% of Adult and Adolescent Patients in the QVAR REDIHALER or QVAR MDI Groups and Greater Than Placebo by Treatment and Daily Dose
Preferred
Term
Number (%) of patients
QVAR REDIHALER
QVAR MDI
Placebo
80 mcg
N=90
160 mcg
N=92
320 mcg
N=214
640 mcg
N=211
320 mcg
N=212
640 mcg
N=107
N=304
Oral Candidiasis
0
2 (2)
7 (3)
15 (7)
6 (3)
9 (8)
1 (<1)
Upper Respiratory Tract Infection
3 (3)
3 (3)
9 (4)
6 (3)
17 (8)
4 (4)
6 (2)
Nasopharyngitis
4 (4)
2 (2)
3 (1)
3 (1)
6 (3)
4 (4)
4 (1)
Oropharyngeal Pain
2 (2)
2 (2)
1 (<1)
3 (1)
6 (3)
4 (4)
2 (<1)
Viral Upper Respiratory Tract Infection
3 (3)
0
1 (<1)
3 (1)
4 (2)
2 (<1)
4 (1)
Sinusitis
3 (3)
0
1 (<1)
2 (<1)
1 (<1)
1 (<1)
2 (<1)
Rhinitis Allergic
0
3 (3)
0
2 (<1)
0
1 (<1)
0
*QVAR MDI=QVAR Inhalation Aerosol
Other adverse reactions that occurred in clinical trials using QVAR REDIHALER with an incidence of 1% to 3% and which occurred at a greater incidence than placebo were back pain, headache, pain, nausea and cough.
Pediatric Patients 4 to 11 Years of Age: The adverse reaction information presented in Table 2 concerning QVAR REDIHALER and QVAR MDI is derived from one 12‑week placebo-controlled study in pediatric patients 4 to 11 years of age with persistent asthma.
Table 2 Adverse Reactions Experienced by at Least 3% of Patients 4 to 11 Years of Age in the QVAR REDIHALER or QVAR MDI Groups and Greater Than Placebo by Treatment and Daily Dose
Preferred Term
Number (%) of patients
QVAR REDIHALER
QVAR MDI
Placebo
80 mcg
N=126
160 mcg N=125
80 mcg
N=125
160 mcg
N=125
N=127
Upper Respiratory Tract Infection
3 (2.4)
1 (0.8)
6 (4.8)
5 (4.0)
5 (3.9)
Nasopharyngitis
5 (4.0)
11 (8.8)
6 (4.8)
6 (4.8)
4 (3.1)
Viral Upper Respiratory Tract Infection
5 (4.0)
5 (4.0)
3 (2.4)
1 (0.8)
4 (3.1)
Pharyngitis
4 (3.2)
4 (3.2)
4 (3.2)
4 (3.2)
2 (1.6)
Cough
1 (0.8)
3 (2.4)
9 (7.2)
6 (4.8)
4 (3.1)
Vomiting
2 (1.6)
2 (1.6)
4 (3.2)
0
2 (1.6)
Headache
2 (1.6)
5 (4.0)
0
4 (3.2)
5 (3.9)
Pyrexia
1 (0.8)
4 (3.2)
4 (3.2)
3 (2.4)
3 (2.4)
*QVAR MDI=QVAR Inhalation Aerosol
Other adverse reactions that occurred in clinical trials using QVAR REDIHALER with an incidence of 1% to 3% and which occurred at a greater incidence than placebo were influenza, gastroenteritis viral, ear infection, oral candidiasis, diarrhea, and myalgia.
6.2 Postmarketing Experience
In addition to the adverse reactions reported from clinical trials with QVAR REDIHALER, the following adverse reactions have been identified during post-approval use of QVAR MDI and other inhaled corticosteroids. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Local Effects: Localized infections with Candida albicans have occurred in patients treated with beclomethasone dipropionate or other orally inhaled corticosteroids [see Warnings and Precautions (5.1)].
Psychiatric and Behavioral Changes: Aggression, depression, sleep disorders, psychomotor hyperactivity, and suicidal ideation have been reported (primarily in children).
Eye Disorders: Blurred vision, central serous chorioretinopathy (CSC).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well‑controlled studies with QVAR REDIHALER or beclomethasone dipropionate in pregnant women. There are clinical considerations with the use of inhaled corticosteroids (ICS), including beclomethasone dipropionate, in pregnant women [see Clinical Considerations]. Also, no published studies, including studies of large birth registries, have to date related the use of ICS to any increases in congenital malformations or other adverse perinatal outcomes. Thus, available human data do not establish the presence or absence of drug‑associated risk to the fetus. In animal reproduction studies, beclomethasone dipropionate resulted in adverse developmental effects in mice and rabbits at subcutaneous doses equal to or greater than approximately 0.75 times the maximum recommended human daily inhalation dose (MRHDID) in adults (0.64 mg/day) [see Data]. In rats exposed to beclomethasone dipropionate by inhalation, dose‑related gross injury to the fetal adrenal glands was observed at doses greater than 180 times the MRHDID, but there was no evidence of external or skeletal malformations or embryolethality at inhalation doses of up to 440 times the MRHDID.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. In the US general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2‑4% and 15‑20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
The risk of complications to the mother and developing fetus from inadequate control of asthma must be balanced against the risks from exposure to beclomethasone dipropionate. In women with poorly or moderately controlled asthma, evidence demonstrates that there is an increased risk of preeclampsia in the mother and prematurity, low birth weight, and small for gestational age for the neonate. The level of asthma control should be closely monitored in pregnant women and treatment adjusted to maintain optimal control.
Labor or Delivery
There are no specific human data regarding any adverse effects of inhaled beclomethasone dipropionate on labor and delivery.
Data
Animal Data
In an embryofetal development study in pregnant rats, beclomethasone dipropionate administration during organogenesis from gestation days 6 to 15 at inhaled doses 180 times the MRHDID in adults and higher (on a mg/m2 basis at maternal doses of 11.5 and 28.3 mg/kg/day) produced dose‑dependent gross injury (characterized by red foci) of the adrenal glands in fetuses. There were no findings in the adrenal glands of rat fetuses at an inhaled dose that was 40 times the MRHDID in adults (on a mg/m2 basis at a maternal dose of 2.4 mg/kg/day). There was no evidence of external or skeletal malformations or embryolethality in rat at inhaled doses up to 440 times the MRHDID (on a mg/m2 basis at maternal doses up to 28.3 mg/kg/day).
In an embryofetal development study in pregnant mice, beclomethasone dipropionate administration from gestation days 1 to 18 at subcutaneous doses equal to and greater than 0.75 times the MRHDID in adults (on a mg/m2 basis at maternal doses of 0.1 mg/kg/day and higher) produced adverse developmental effects (increased incidence of cleft palate). A no-effect dose in mice was not identified. In a second embryofetal development study in pregnant mice, beclomethasone dipropionate administration from gestation days 1 to 13 at subcutaneous doses equal to and greater than 2.3 times the MRHDID in adults (on a mg/m2 basis at a maternal dose of 0.3 mg/kg/day) produced embryolethal effects (increased fetal resorptions) and decreased pup survival.
In an embryofetal development study in pregnant rabbits, beclomethasone dipropionate administration during organogenesis from gestation days 7 to 16 at subcutaneous doses equal to and greater than 0.75 times the MRHDID in adults (on a mg/m2 basis at maternal doses of 0.025 mg/kg/day and higher) produced external and skeletal malformations and embryolethal effects (increased fetal resorptions). There were no effects in fetuses of pregnant rabbits administered a subcutaneous dose 0.2 times the MRHDID in adults (on a mg/m2 basis at a maternal dose of 0.006 mg/kg/day).
8.2 Lactation
Risk Summary
There are no data available on the presence of beclomethasone dipropionate in human milk, the effects on the breastfed child, or the effects on milk production. However, other inhaled corticosteroids have been detected in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for QVAR REDIHALER and any potential adverse effects on the breastfed child from beclomethasone dipropionate or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Impairment of fertility was observed in rats and dogs at oral doses of beclomethasone dipropionate corresponding to 250 and 25 times the MRHDID for adults on a mg/m2 basis, respectively. [see Nonclinical Toxicology(13.1)].
8.4 Pediatric Use
Five-hundred and one children between the ages of 4 and 11 were treated with at least one dose of QVAR REDIHALER or QVAR MDI in one 12‑week clinical trial. The safety and effectiveness of QVAR REDIHALER in children below 4 years of age have not been established.
Do not use QVAR REDIHALER with a spacer or volume holding chamber.
Controlled clinical studies have shown that inhaled corticosteroids may cause a reduction in growth velocity in pediatric patients. A 12‑month, randomized, controlled clinical trial evaluated the effects of QVAR MDI versus beclomethasone dipropionate in a CFC propellant‑based formulation (CFC‑BDP) on growth in children age 5 to 11. A total of 520 patients were enrolled, of whom 394 received QVAR MDI (100 to 400 mcg/day ex‑valve) and 126 received CFC‑BDP (200 to 800 mcg/day ex‑valve). Similar control of asthma was noted in each treatment arm. When comparing results at month 12 to baseline, the mean growth velocity in children treated with QVAR MDI was approximately 0.5 cm/year less than that noted with children treated with CFC‑BDP via large‑volume spacer. The long‑term effects of the reduction in growth velocity associated with orally inhaled corticosteroids, including the impact on final adult height, are unknown. The potential for "catch‑up" growth following discontinuation of treatment with orally inhaled corticosteroids has not been adequately studied.
The growth of children and adolescents receiving orally inhaled corticosteroids, including QVAR REDIHALER, should be monitored routinely (e.g., via stadiometry). If a child or adolescent on any corticosteroid appears to have growth suppression, the possibility that he/she is particularly sensitive to this effect should be considered. The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the risks associated with alternative therapies. To minimize the systemic effects of orally inhaled corticosteroids, including QVAR REDIHALER, each patient should be titrated to his/her lowest effective dose [see Dosage and Administration (2.2)].
8.5 Geriatric Use
Clinical studies of QVAR REDIHALER did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
11 DESCRIPTION
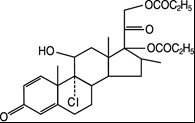
The active component of QVAR REDIHALER 40 mcg Inhalation Aerosol and QVAR REDIHALER 80 mcg Inhalation Aerosol is beclomethasone dipropionate, USP, a corticosteroid having the chemical name 9-chloro-11ß,17,21-trihydroxy-16ß-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate. Beclomethasone dipropionate is a diester of beclomethasone, a synthetic corticosteroid chemically related to dexamethasone. Beclomethasone differs from dexamethasone in having a chlorine at the 9‑alpha carbon in place of a fluorine, and in having a 16‑beta-methyl group instead of a 16‑alpha-methyl group. Beclomethasone dipropionate is a white to creamy white, odorless powder with a molecular formula of C28H37ClO7 and a molecular weight of 521.1. Its chemical structure is:
QVAR REDIHALER is a pressurized, breath‑actuated, metered‑dose aerosol with a dose counter intended for oral inhalation only. Each unit consists of a sealed breath‑actuated inhaler device enclosing a canister containing a solution of beclomethasone dipropionate in propellant HFA‑134a (1,1,1,2 tetrafluoroethane) and ethanol (0.85 g). QVAR REDIHALER 40 mcg delivers 40 mcg of beclomethasone dipropionate from the actuator mouthpiece and 50 mcg from the canister valve. QVAR REDIHALER 80 mcg delivers 80 mcg of beclomethasone dipropionate from the actuator mouthpiece and 100 mcg from the canister valve. Both products deliver 50 microliters (59 milligrams) of solution formulation as an aerosol from the canister valve with each actuation. The 40‑mcg canisters and the 80‑mcg canisters provide 120 inhalations each. Since the QVAR REDIHALER canister is fitted with a primeless valve, no priming actuations are required before use. For both products, an actuation was always triggered by a 20 L/min inspiratory flow rate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Beclomethasone dipropionate is a corticosteroid demonstrating potent anti‑inflammatory activity. The precise mechanism of corticosteroid action on asthma is not known. Corticosteroids have been shown to have multiple anti‑inflammatory effects, inhibiting both inflammatory cells (e.g., mast cells, eosinophils, basophils, lymphocytes, macrophages, and neutrophils) and release of inflammatory mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines). These anti‑inflammatory actions of corticosteroids contribute to their efficacy in asthma.
Beclomethasone dipropionate is a prodrug that is rapidly activated by hydrolysis to the active monoester, 17‑monopropionate (17‑BMP). Beclomethasone‑17‑monopropionate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor which is approximately 13 times that of dexamethasone, 6 times that of triamcinolone acetonide, 1.5 times that of budesonide and 25 times that of beclomethasone dipropionate. The clinical significance of these findings is unknown.
Studies in patients with asthma have shown a favorable ratio between topical anti‑inflammatory activity and systemic corticosteroid effects with recommended doses of QVAR REDIHALER.
12.2 Pharmacodynamics
HPA Axis Effects
The effects of QVAR MDI on the hypothalamic‑pituitary‑adrenal (HPA) axis were studied in 40 corticosteroid-naive patients. QVAR MDI, at doses of 80, 160, or 320 mcg twice daily, was compared with placebo and 336 mcg twice daily of CFC‑BDP. Active treatment groups showed an expected dose‑related reduction in 24‑hour urinary-free cortisol (a sensitive marker of adrenal production of cortisol). Patients treated with the highest dose recommended of QVAR MDI (320 mcg twice daily) had a 37.3% reduction in 24‑hour urinary‑free cortisol compared to a reduction of 47.3% produced by treatment with 336 mcg twice daily of CFC‑BDP. There was a 12.2% reduction in 24‑hour urinary‑free cortisol seen in the group of patients that received 80 mcg twice daily of QVAR MDI and a 24.6% reduction in the group of patients that received 160 mcg twice daily. An open label study of 354 asthma patients given QVAR MDI at recommended doses for one year assessed the effect of treatment with this product on the HPA axis (as measured by both morning and stimulated plasma cortisol). Less than 1% of patients treated for one year with this product had an abnormal response (peak less than 18 mcg/dL) to a short-cosyntropin test.
12.3 Pharmacokinetics
Beclomethasone dipropionate undergoes rapid and extensive conversion to beclomethasone‑17‑monopropionate (17‑BMP) during absorption. The pharmacokinetics of beclomethasone dipropionate and 17‑BMP were studied in subjects given single doses.
Absorption
The mean peak plasma concentration (Cmax) of BDP was 6635 pg/mL at 2 minutes after inhalation of 320 mcg using QVAR REDIHALER (4 inhalations of the 80 mcg/inhalation strength). The mean peak plasma concentration of the major and most active metabolite, 17-BMP, was 1464 pg/mL at 10 minutes after inhalation of 320 mcg of QVAR REDIHALER.
Distribution
The in vitro protein binding for 17‑BMP was reported to be 94‑96% over the concentration range of 1000 to 5000 pg/mL. Protein binding was constant over the concentration range evaluated. There is no evidence of tissue storage of beclomethasone dipropionate or its metabolites.
Elimination
The major route of elimination of inhaled beclomethasone dipropionate appears to be via hydrolysis. More than 90% of inhaled beclomethasone dipropionate is found as 17‑BMP in the systemic circulation. The mean terminal half‑life of 17‑BMP is approximately 4 hours for QVAR REDIHALER.
Metabolism
Three major metabolites are formed via esterases:
- beclomethasone‑17‑monopropionate (17‑BMP)
- beclomethasone‑21‑monopropionate (21‑BMP)
- beclomethasone (BOH)
Lung slices metabolize beclomethasone dipropionate rapidly to 17‑BMP and more slowly to BOH. 17‑BMP is the most active metabolite.
Excretion
Irrespective of the route of administration (injection, oral or inhalation), beclomethasone dipropionate and its metabolites are mainly excreted in the feces. Less than 10% of the drug and its metabolites are excreted in the urine.
Specific Populations
Age: No pharmacokinetic studies for QVAR REDIHALER have been conducted in neonates or elderly subjects.
Pediatrics: No pharmacokinetic studies for QVAR REDIHALER have been conducted in pediatric subjects aged of 4 to 17 years. However, the pharmacokinetics of 17‑BMP, including dose and strength proportionalities, is similar in children and adults using QVAR MDI, although the exposure is highly variable. In 17 children (mean age 10 years), the Cmax of 17‑BMP was 787 pg/mL at 0.6 hour after inhalation of 160 mcg (4 actuations of the 40 mcg/actuation strength of QVAR MDI). The systemic exposure to 17‑BMP from 160 mcg of QVAR MDI administered without a spacer was comparable to the systemic exposure to 17‑BMP from 336 mcg CFC‑BDP administered with a large volume spacer in 14 children (mean age 12 years). This implies that approximately twice the systemic exposure to 17‑BMP would be expected for comparable mg doses of QVAR MDI without a spacer and CFC‑BDP with a large volume spacer.
Sex: The influence of sex on the pharmacokinetics of QVAR REDIHALER has not been studied.
Race: The influence of race on the pharmacokinetics of QVAR REDIHALER has not been studied.
Renal Impairment: The effect of renal impairment on the pharmacokinetics of QVAR REDIHALER has not been evaluated.
Hepatic Impairment: The effect of hepatic impairment on the pharmacokinetics of QVAR REDIHALER has not been evaluated.
Drug Interaction Studies: In vitro and in vivo drug interaction studies have not been conducted with QVAR REDIHALER.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenicity of beclomethasone dipropionate was evaluated in rats which were exposed for a total of 95 weeks, 13 weeks at inhalation doses up to 0.4 mg/kg/day and the remaining 82 weeks at combined oral and inhalation doses up to 2.4 mg/kg/day. There was no evidence of treatment‑related increases in the incidence of tumors in this study at the highest dose, which is approximately 37 and 72 times the MRHDID in adults and children, respectively, on a mg/m2 basis.
Beclomethasone dipropionate did not induce gene mutation in bacterial cells or mammalian Chinese hamster ovary (CHO) cells in vitro. No significant clastogenic effect was seen in cultured CHO cells in vitro or in the mouse micronucleus test in vivo.
In rats, beclomethasone dipropionate caused decreased conception rates at an oral dose of 16 mg/kg/day (approximately 250 times the MRHDID in adults on a mg/m2 basis). Impairment of fertility, as evidenced by inhibition of the estrous cycle in dogs, was observed following treatment by the oral route at a dose of 0.5 mg/kg/day (approximately 25 times the MRHDID in adults on a mg/m2 basis). No inhibition of the estrous cycle in dogs was seen following 12 months of exposure to beclomethasone dipropionate by the inhalation route at an estimated daily dose of 0.33 mg/kg (approximately 17 times the MRHDID in adults on a mg/m2 basis).
-
14 CLINICAL STUDIES
The safety and efficacy of QVAR REDIHALER were evaluated in 1,858 patients with asthma. The development program included 2 confirmatory trials of 12 weeks duration and 1 confirmatory trial of 6 weeks duration in patients 12 years of age and older, and 1 confirmatory trial of 12 weeks duration in patients 4 to 11 years of age. The efficacy of QVAR REDIHALER is based primarily on the confirmatory trials described below.
14.1 Trials in the Maintenance Treatment of Asthma
Adult and Adolescent Patients 12 Years of Age and Older
Two confirmatory Phase 3 clinical trials were conducted comparing QVAR REDIHALER with placebo in adult and adolescent patients with persistent asthma (Trial 1 and Trial 2).
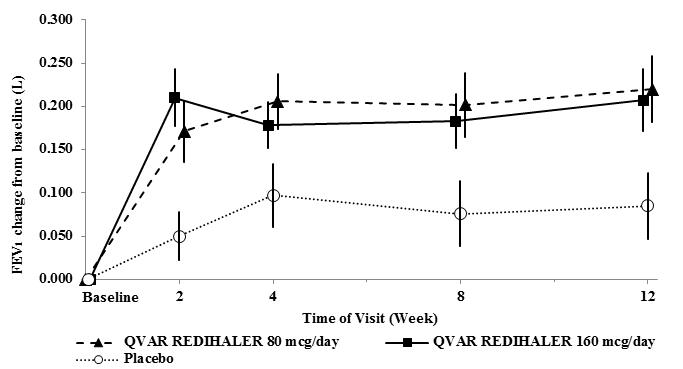
Trial 1(NCT02040779): This randomized, double-blind, parallel-group, placebo-controlled, 12-week, efficacy and safety trial compared QVAR REDIHALER 40 and 80 mcg given as 1 inhalation twice daily with placebo in adult and adolescent patients with persistent symptomatic asthma despite low-dose inhaled corticosteroid or non-corticosteroid asthma therapy. Patients aged 12 years and older who met the entry criteria including FEV1 40-85 percent of predicted normal, reversible bronchoconstriction of 15% with short-acting inhaled beta-agonist entered a 14-21 day run-in period. 270 patients (104 previously treated with inhaled corticosteroids) who met all the randomization criteria including asthma symptoms and rescue medication use were discontinued from asthma maintenance medication and randomized equally to treatment with QVAR REDIHALER 80 mcg/day, QVAR REDIHALER 160 mcg/day or placebo. Baseline FEV1 values were similar across treatments. The primary endpoint for this trial was the standardized baseline-adjusted trough morning forced expiratory volume in 1 second (FEV1) area under the effect curve from time zero to 12 weeks [FEV1 AUEC(0-12wk)]. Patients in both treatment groups had significantly greater improvements in trough FEV1 compared to placebo (QVAR REDIHALER 80 mcg/day, LS mean change of 0.124 L and QVAR REDIHALER 160 mcg/day, LS mean change of 0.116 L over 12 weeks) (Table 3).
In addition, the mean change from baseline is displayed in Figure 1. Both doses of QVAR REDIHALER were effective in improving asthma control with significantly greater improvements in FEV1 and morning PEF when compared to placebo. Reduction in asthma symptoms was also supportive of the efficacy of QVAR REDIHALER.
Figure 1: A 12‑ Week Clinical Trial in Patients with Asthma: Mean Change in FEV1
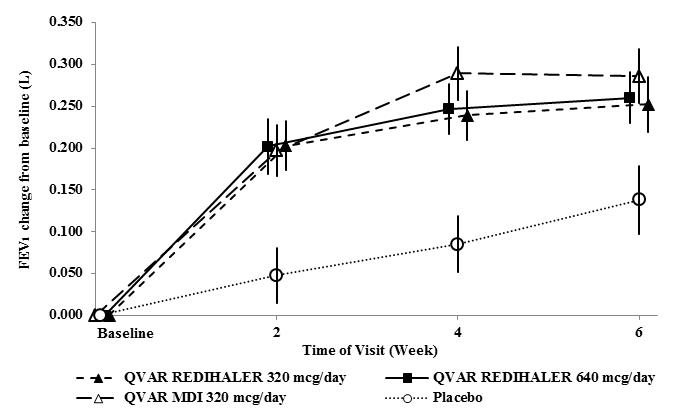
Trial 2 (NCT02513160): This randomized, double-blind, parallel-group, placebo-controlled, 6-week, efficacy and safety trial compared QVAR REDIHALER 40 and 80 mcg given as 4 inhalations twice daily and placebo in adult and adolescent patients with persistent symptomatic asthma despite treatment with non-corticosteroid, inhaled corticosteroids (with or without a long acting beta agonist [LABA]), or combination asthma therapy. The study also included a reference treatment group, QVAR® Inhalation Aerosol (QVAR MDI) 40 mcg, 4 inhalations twice daily. Patients aged 12 years and older who met the entry criteria including FEV1 50-90% predicted normal, reversible bronchoconstriction of at least 10% with short-acting inhaled beta-agonist discontinued baseline asthma treatment and entered a 2-4 week run-in period. 425 patients (257 previously treated with ICS with or without LABA) who met all the randomization criteria including FEV1 of 40-85% predicted and 15% reversibility with short-acting inhaled beta-agonist, and asthma symptoms were randomized equally to QVAR REDIHALER 320 mcg/day, QVAR REDIHALER 640 mcg/day, QVAR MDI 320 mcg/day or placebo. Baseline FEV1 values were similar across treatments. The primary endpoint for this trial was the standardized baseline-adjusted trough morning forced expiratory volume in 1 second (FEV1) area under the effect curve from time zero to 6 weeks [FEV1 AUEC(0-6wk)]. Patients in both treatment groups had significantly greater improvements in trough FEV1 compared to placebo (QVAR REDIHALER 320 mcg/day, LS mean change of 0.144 L and QVAR REDIHALER 640 mcg/day, LS mean change of 0.150 L over 6 weeks) (Table 3). Treatment with QVAR MDI was similar. The change from baseline in morning FEV1 during the trial is displayed in Figure 2. Both doses of QVAR REDIHALER were effective in improving asthma control with significantly greater improvements in FEV1, morning PEF, weekly average of daily trough morning FEV1, reduced rescue medication use and improved asthma symptom scores than with placebo. Similar results were demonstrated with QVAR MDI.
Figure 2: A 6‑ Week Dose Response Clinical Trial in Patients with Inhaled Corticosteroid-Dependent Asthma: Mean Change in FEV1 as Percent of Predicted
Side-by-side comparison of the primary analysis of standardized baseline-adjusted trough morning FEV1 from time zero to the end of the treatment period for both studies is shown below in Table 3.
Table 3: Primary Analysis of Standardized Baseline-Adjusted Trough Morning FEV1 (L) AUEC from Time Zero to the End of the Treatment Period 12-week Study and 6-week Dose Response Study
12 weeks; FAS
6 weeks; mITT Analysis set
Parameter
Statistic
Placebo
(N=90)QVAR REDIHALER
80 mcg/day
(N=88)QVAR REDIHALER
160 mcg/day
(N=92)Placebo
(N=107)QVAR REDIHALER
320 mcg/day
(N=108)QVAR REDIHALER
640 mcg/day
(N=105)QVAR MDI *
320 mcg/day
(N=105)Difference from placebo
Difference of Least Square mean
—
0.124
0.116
—
0.144
0.150
0.148
95% CI
—
0.054, 0.193
0.048, 0.185
—
0.0807, 0.2066
0.0868, 0.2132
0.0847, 0.2114
*QVAR MDI=QVAR Inhalation Aerosol
Pediatric Patients 4 to 11 Years of Age
This randomized, double-blind, parallel-group, placebo controlled, 12-week, global efficacy and safety trial (NCT02040766) compared QVAR REDIHALER 40 or 80 mcg, QVAR MDI 40 or 80 mcg or placebo given as 1 inhalation twice daily in pediatric patients aged 4 through 11 years old with persistent symptomatic asthma despite treatment with non-corticosteroid or low dose inhaled corticosteroid (with or without a long acting beta agonist [LABA]). Patients aged 4 to 5 years who were technically unable to complete spirometry participated in the safety population. Patients who met the entry criteria including FEV1 40-90% predicted normal and reversible bronchoconstriction of at least 12% with short acting inhaled beta agonist entered a 14-21 day run in period. Patients who met the randomization criteria including asthma symptoms and rescue medication use discontinued asthma therapy and were randomized equally across treatment groups. Five hundred sixty-eight (568) pediatric patients with symptomatic asthma of which 410 had previously been treated with low dose inhaled corticosteroids with or without a LABA were randomized to receive either 40 mcg or 80 mcg twice daily of QVAR REDIHALER, QVAR MDI or placebo. The primary endpoint was the change from baseline in trough percent predicted FEV1 AUEC (0-12 weeks). While the primary endpoint, was not statistically significant, change in weekly average of daily morning peak expiratory flow (PEF, L/min) over the 12 week treatment period was 11.3 [95% CI: 5.58, 17.06] and 8.5 [95% CI: 2.71, 14.24] for the 80 mcg/day and 160 mcg/day doses of QVAR REDIHALER, respectively, at nominal significance. Similar results were seen with evening PEF.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA‑Approved Patient Labeling (Patient Information and Instructions for Use).
Patients should be given the following information:
Local Effects
Inform patients that localized infections with Candida albicans occurred in the mouth and pharynx in some patients. If oropharyngeal candidiasis develops, treat it with appropriate local or systemic (i.e., oral) antifungal therapy while still continuing therapy with QVAR REDIHALER, but at times therapy with QVAR REDIHALER may need to be temporarily interrupted under close medical supervision. Rinsing the mouth with water without swallowing after inhalation is advised to help reduce the risk of thrush.
Status Asthmaticus and Acute Asthma Symptoms
Inform patients that QVAR REDIHALER is not a bronchodilator and is not intended for use as rescue medicine for acute asthma exacerbations. Advise patients to treat acute asthma symptoms with an inhaled, short‑acting beta2‑agonist such as albuterol. Instruct the patient to contact their physicians immediately if there is deterioration of their asthma.
Immunosuppression
Warn patients who are on immunosuppressant doses of corticosteroids to avoid exposure to chickenpox or measles and, if exposed, to consult their physicians without delay. Inform patients of potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
Hypercorticism and Adrenal Suppression
Advise patients that QVAR REDIHALER may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, instruct patients that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Patients should taper slowly from systemic corticosteroids if transferring to QVAR REDIHALER.
Immediate Hypersensitivity Reactions
Advise patients that immediate hypersensitivity reactions (e.g., urticaria, angioedema, rash, bronchospasm, and hypotension), including anaphylaxis, may occur after administration of QVAR REDIHALER. Patients should discontinue QVAR REDIHALER if such reactions occur and contact their healthcare provider or get emergency medical help.
Reduction in Bone Mineral Density
Advise patients who are at an increased risk for decreased BMD that the use of corticosteroids may pose an additional risk.
Reduced Growth Velocity
Inform patients that orally inhaled corticosteroids, including QVAR REDIHALER, may cause a reduction in growth velocity when administered to pediatric patients. Physicians should closely follow the growth of adolescents taking corticosteroids by any route.
Ocular Effects
Long-term use of inhaled corticosteroids may increase the risk of some eye problems (cataracts, glaucoma or blurred vision); consider regular eye examinations.
Pregnancy
Inform patients who are pregnant or nursing that they should contact their physician about the use of QVAR REDIHALER.
Use Daily for Best Effect
Patients should use QVAR REDIHALER at regular intervals as directed. The daily dosage of QVAR REDIHALER should not exceed 8 inhalations per day. Advise patients, if they miss a dose, to take their next dose at the same time they normally do. Individual patients will experience a variable time to onset and degree of symptom relief and the full benefit may not be achieved until treatment has been administered for 1 to 2 weeks or longer. Patients should not increase the prescribed dosage but should contact their physicians if symptoms do not improve or if the condition worsens. Instruct patients to not stop use of QVAR REDIHALER abruptly. Patients should contact their physicians immediately if they discontinue use of QVAR REDIHALER.
Caring for and Storing the Inhaler
For normal hygiene, the mouthpiece of QVAR REDIHALER should be cleaned weekly with a clean, dry tissue or cloth. Never wash or put any part of QVAR REDIHALER in water. Patient should replace QVAR REDIHALER if washed or placed in water.
Instruct patients to store the inhaler at room temperature and to avoid exposure to extreme heat and cold.
Inform patients that shaking the inhaler prior to use is not necessary. Instruct patients not to shake the inhaler with the cap open to avoid possible actuation of the device.
Instruct patients to never take QVAR REDIHALER apart.
Inform patients that QVAR REDIHALER has a dose counter attached to the actuator at the rear of the mouth piece. When the patient receives the inhaler, the number 120 will be displayed. The dose counter will count down each time a spray is released. The dose-counter window displays the number of sprays left in the inhaler in units of two (e.g., 120, 118, 116, etc). When the counter displays 20, the color of the numbers will change to red to remind the patient to contact their pharmacist for a refill of medication or consult their healthcare provider for a prescription refill. When the dose counter reaches 0, the background will change to solid red. Inform patients to discard QVAR REDIHALER when the dose counter displays 0 or after the expiration date on the product, whichever comes first.
Rx only
Marketed by: Teva Respiratory, LLC
Frazer, PA 19355
Developed and Manufactured by:
Norton (Waterford) Limited
Unit 301, IDA Industrial Park, Cork Road, Waterford, Ireland
© 2018 Teva Respiratory, LLC
U.S. Patent 7,637,260; 8,132,712; 8,931,476
QVARH-002

-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
QVAR REDIHALER (kue' var red-ee-haye' ler)
(beclomethasone dipropionate HFA) inhalation aerosol
What is QVAR REDIHALER?
QVAR REDIHALER is a breath‑actuated inhaled prescription medicine used as a maintenance treatment for the prevention and control of asthma in people 4 years of age and older.
- QVAR REDIHALER is not used to relieve sudden breathing problems.
It is not known if QVAR REDIHALER is safe and effective in children less than 4 years of age.
Who should not use QVAR REDIHALER?
Do not use QVAR REDIHALER:
- to treat sudden severe symptoms of asthma.
- as a rescue inhaler.
- if you are allergic to beclomethasone dipropionate or any of the ingredients in QVAR REDIHALER. See the end of this leaflet for a complete list of ingredients in QVAR REDIHALER.
What should I tell my healthcare provider before using QVAR REDIHALER?
Before using QVAR REDIHALER, tell your healthcare provider about all of your medical conditions, including if you:
- are exposed to chickenpox or measles.
- have or have had tuberculosis (TB) or any untreated fungal, bacterial or viral infections, or eye infections caused by herpes.
- have weak bones (osteoporosis).
- have an immune system problem.
- have or have had eye problems, such as blurred vision, increased pressure in your eye (glaucoma) or cataracts.
- are pregnant or plan to become pregnant. It is not known if QVAR REDIHALER will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if QVAR REDIHALER passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you use QVAR REDIHALER.
Tell your healthcare provider about all of the medications you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use QVAR REDIHALER?
Read the step-by-step instructions for using QVAR REDIHALER at the end of this Patient Information leaflet.
- Use QVAR REDIHALER exactly as your healthcare provider tells you to. Do not use QVAR REDIHALER more often than it is prescribed.
- Do not shake the inhaler before using it. Especially, do not shake the inhaler with the cap open. This could cause the device to accidentally release medicine before you are ready to take it.
- You do not need to prime QVAR REDIHALER.
- If your child needs to use QVAR REDIHALER, watch your child closely to make sure your child uses the inhaler correctly.
- Do not change or stop using QVAR REDIHALER or other asthma medicines used to treat your breathing problems unless your healthcare provider tells you to. Your healthcare provider will change your medicines as needed.
- You must use QVAR REDIHALER regularly. It may take 2 to 4 weeks, or longer, after you start using QVAR REDIHALER for your asthma symptoms to get better. Do not stop using QVAR REDIHALER, even if you are feeling better, unless your healthcare provider tells you to.
- QVAR REDIHALER comes in 2 strengths (40 and 80 mcg). Your healthcare provider has prescribed the strength that is best for you. Pay attention to the differences between QVAR REDIHALER and your other inhaled medicines, including their prescribed use and the way they look.
- QVAR REDIHALER does not relieve sudden asthma symptoms. Always have a rescue inhaler with you to treat sudden symptoms. Use your rescue inhaler if you have breathing problems between doses of QVAR REDIHALER. If you do not have a rescue inhaler, call your healthcare provider to have a rescue inhaler prescribed for you.
- Rinse your mouth with water without swallowing after each dose of QVAR REDIHALER. This will help lessen the chance of getting a yeast infection (thrush) in your mouth and throat.
- Do not spray QVAR REDIHALER in your face or eyes. If you accidentally get QVAR REDIHALER in your eyes, rinse your eyes with water and if redness or irritation continues, call your healthcare provider.
What should I avoid while taking QVAR REDIHALER?
If you have not had, or have not been vaccinated against, chickenpox or measles, you should stay away from people who are infected.
What are the possible side effects of QVAR REDIHALER?
QVAR REDIHALER may cause serious side effects, including:
- fungal infections (thrush) in your mouth and throat. You may develop a yeast infection (Candida albicans) in your mouth and throat.Tell your healthcare provider if you have any redness or white colored patches in your mouth or throat. Rinse your mouth with water without swallowing after using QVAR REDIHALER to help prevent an infection in your mouth or throat.
- worsening asthma or sudden asthma attacks. You should contact your healthcare provider right away if you do not get relief from your sudden asthma attacks, after using your rescue inhaler, during your treatment with QVAR REDIHALER.
- reduced adrenal function (adrenal insufficiency). Adrenal insufficiency that can lead to death can happen when you stop taking oral corticosteroid medicines and start using inhaled corticosteroid medicines. Adrenal insufficiency can also happen in people who take higher doses of QVAR REDIHALER than recommended over a long period of time. When your body is under stress such as from fever, trauma (such as a car accident), infection, or surgery, adrenal insufficiency can get worse. Signs and symptoms of adrenal insufficiency may include:
○ feeling tired or exhausted (fatigue) ○ lack of energy ○ low blood pressure (hypotension)
○ dizziness or feeling faint ○ nausea and vomiting ○ weakness
- immune system effects and a higher chance for infections. Tell your healthcare provider about any signs or symptoms of infection such as:
○ fever ○ chills ○ pain ○ feeling tired
○ body aches ○ nausea ○ vomiting
- increased wheezing (bronchospasm) right after using QVAR REDIHALER. Always have a rescue inhaler with you to treat sudden wheezing.
- serious allergic reactions. Stop using QVAR REDIHALER and call your healthcare provider or get emergency medical help right away if you get any of the following signs or symptoms of a serious allergic reaction:
○ hives ○ swelling of your lips, tongue or face ○ rash ○ breathing problems
- slowed growth in children. Children should have their growth checked regularly while using QVAR REDIHALER.
- lower bone density. This may be a problem for people who already have a higher chance for low bone density (osteoporosis).
- eye problems. If you have had glaucoma, cataracts or blurred vision in the past, you should have regular eye exams while using QVAR REDIHALER.
The most common side effects of QVAR REDIHALER include:
- yeast infection in the mouth (oral candidiasis)
- cold symptoms (upper respiratory tract infection)
- pain in the throat (oropharyngeal pain)
- pain or swelling in your nose and throat (nasopharyngitis)
- sinus irritation (sinusitis)
- hay fever (allergic rhinitis)
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of QVAR REDIHALER. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store QVAR REDIHALER?
- Store QVAR REDIHALER at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- Your QVAR REDIHALER canister should only be used with the QVAR REDIHALER actuator. Do not use any other medicines in your QVAR REDIHALER actuator.
- The contents of your QVAR REDIHALER canister are under pressure. Do not puncture the QVAR REDIHALER canister.
- Do not store your QVAR REDIHALER canister near heat or a flame. Temperatures above 120ºF may cause the canister to burst.
- Do not throw your QVAR REDIHALER canister into a fire or incinerator.
Keep QVAR REDIHALER and all medicines out of the reach of children.
General information about the safe and effective use of QVAR REDIHALER.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use QVAR REDIHALER for a condition for which it was not prescribed. Do not give QVAR REDIHALER to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about QVAR REDIHALER that is written for health professionals.
What are the ingredients in QVAR REDIHALER?
Active ingredient: beclomethasone dipropionate
Inactive ingredients: propellant HFA-134a and ethanol
For more information, go to www.QVAR.com or call 1-888-483-8279.
QVARHPIL-003
Rev. 3/2018
Instructions for Use
QVAR REDIHALER (kue' var red-ee-haye’ler)
(beclomethasone dipropionate HFA)
inhalation aerosol
Your QVAR REDIHALER Inhaler
OVERVIEW
When you are ready to use your QVAR REDIHALER for the first time, remove the inhaler from the carton.
Important information:
- There is no button. You must close the white cap to prepare the inhaler with medicine before each inhalation.
- Do not shake. This breath-actuated device does not need to be shaken. This is not a press-and-breathe inhaler
- QVAR REDIHALER does not need priming.
- Do not use a spacer or volume holding chamber with QVAR REDIHALER.
- Always use the inhaler in the upright position (with the mouthpiece down).
- Once prepared, the inhaler will deliver 1 inhalation of medicine when you breathe in (inhale) through the mouthpiece. Your dose might require more than 1 inhalation.
- Do not open the white cap or leave it open unless you are ready for your next inhalation. If the cap has been opened for more than 2 minutes or left in the open position, you will need to close the white cap before use.
- Do not suddenly stop using your QVAR REDIHALER. Contact your healthcare provider immediately if you stop using your QVAR REDIHALER.
There are 2 main parts of your QVAR REDIHALER including:
- the inhaler body with the mouthpiece. See Figure A.
- the white cap that covers the mouthpiece of the inhaler. See Figure A.
Figure A
About the Dose Counter
There is a dose counter in the back of the inhaler with a viewing window that shows you how many inhalations of medicine you have left. See Figure B.
- Your QVAR REDIHALER contains 120 inhalations. See Figure B.
- The counter on the back of your inhaler shows how many inhalations you have left.
- When there are 20 inhalations left, the numbers in the dose counter will change to red and you should refill your prescription or ask your healthcare provider for another prescription.
- When the dose counter shows ‘0’, the background will turn solid red and your inhaler is empty. You should stop using the inhaler and throw it away. Donot put your inhaler into a fire or incinerator. See Figure B.
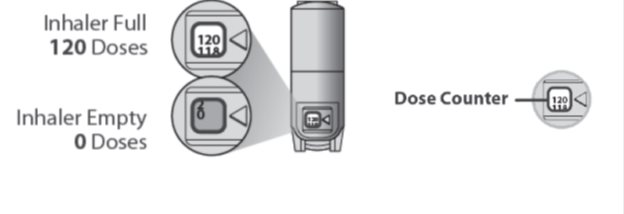
Figure B
Important:
- The white cap must be closed to prepare the inhaler before each inhalation or you will not receive your medicine. See Figure C.
- If the white cap is open, close the white cap to prepare your inhaler and look at the dose counter window to make sure that your inhaler is not empty. See Figure B.
- Do not open the cap until you are ready to take your inhalation.
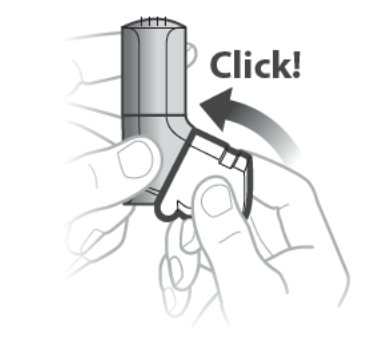
Figure C
Using your QVAR REDIHALER:
Step 1. Open the white cap
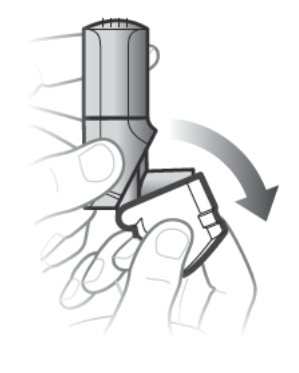
- Open the white cap. See Figure D.
- Breathe out fully.
Figure D
Remember:
- Do not open the cap until you are ready to take your inhalation.
- Never breathe out into the inhaler mouthpiece.
Step 2. Inhale 1 Time
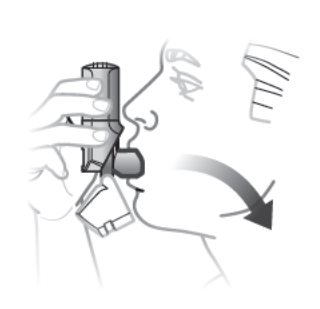
- Place the mouthpiece in your mouth and close your lips around it so you form a good seal.
- Inhale deeply to release medication.
- Remove inhaler, hold breath for 5 to 10 seconds, then, breathe out slowly, away from the inhaler.
Figure E
Remember:
- Hold inhaler upright as you take your inhalation. See Figure E.
Step 3.Close the white cap
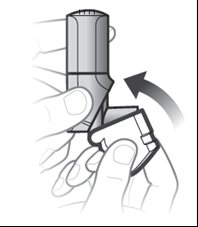
- Close the white cap after inhaling to prepare your next inhalation. See Figure F.
Figure F
If your healthcare provider has told you to take more than 1 inhalation per dose, make sure the white cap is closed and repeat steps 1-3.
After taking your prescribed number of inhalations, rinse your mouth with water without swallowing to help reduce the risk of a fungal infection (thrush) in your mouth.
How to store your QVAR REDIHALER
- Store QVAR REDIHALER at room temperature between 68 ºF to 77ºF (20ºC - 25ºC). Excursions between 59ºF and 86ºF (15ºC and 30ºC) are permitted. Do not use or store near heat or open flame. Exposure to temperatures above 120ºF (49ºC) may cause the canister to burst. Do not throw QVAR REDIHALER into fire or an incinerator. Keep the white cap on the inhaler closed during storage.
- Keep your QVAR REDIHALER inhaler dry and clean at all times.
- Keep your QVAR REDIHALER and all medicines out of the reach of children.
- Throw away QVAR REDIHALER when the dose counter displays ‘0,’ or after the expiration date on the package, whichever comes first.
Cleaning your QVAR REDIHALER
- Do not wash or put any part of your QVAR REDIHALER in water.
- Clean the mouthpiece of your QVAR REDIHALER weekly with a clean, dry tissue or cloth.
Support
- If you have any questions about QVAR REDIHALER or how to use your inhaler, go to www.QvarRedihaler.com or call 1-888-483-8279.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Marketed by: Teva Respiratory, LLC, Frazer, PA 19355; Manufactured by: Norton (Waterford) Limited, Ireland; Copyright ©2018, Teva Respiratory, LLC; All rights reserved .

QVARHIFU-002
Rev.3/2018
- Qvar Redihaler
-
INGREDIENTS AND APPEARANCE
QVAR REDIHALER
beclomethasone dipropionate hfa aerosol, meteredProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50090-3459(NDC:59310-304) Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BECLOMETHASONE DIPROPIONATE (UNII: 5B307S63B2) (BECLOMETHASONE - UNII:KGZ1SLC28Z) BECLOMETHASONE DIPROPIONATE 80 ug Inactive Ingredients Ingredient Name Strength NORFLURANE (UNII: DH9E53K1Y8) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50090-3459-0 1 in 1 CARTON 05/21/2018 1 1 in 1 INHALER 1 120 in 1 CANISTER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA207921 02/15/2018 Labeler - A-S Medication Solutions (830016429) Establishment Name Address ID/FEI Business Operations A-S Medication Solutions 830016429 RELABEL(50090-3459)
Trademark Results [QVAR REDIHALER]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 QVAR REDIHALER 97872541 not registered Live/Pending |
IVAX LLC 2023-04-04 |
 QVAR REDIHALER 86433534 5438539 Live/Registered |
Ivax LLC 2014-10-24 |
 QVAR REDIHALER 86364210 5433697 Live/Registered |
IVAX, LLC 2014-08-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
