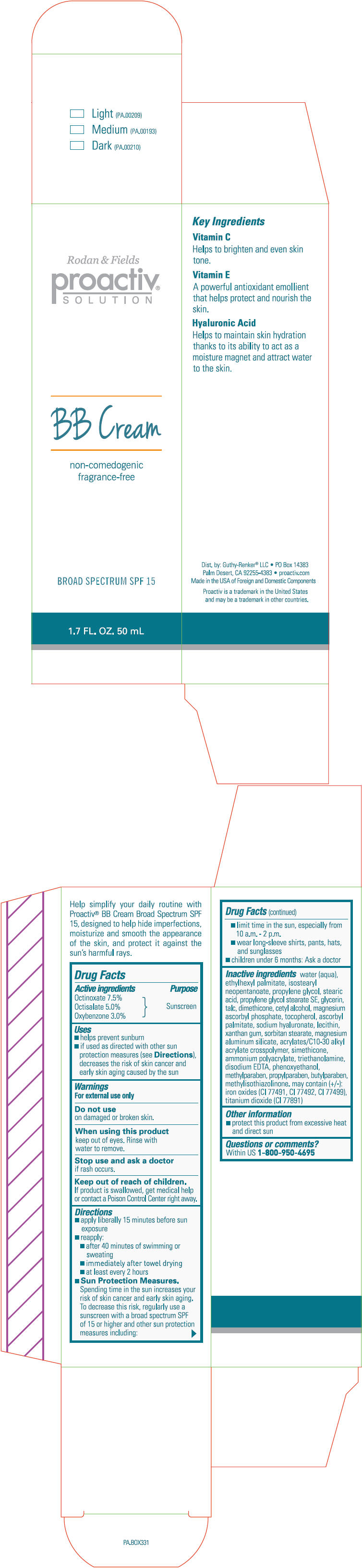
Proactiv Solution BB Cream Broad Spectrum SPF 15 (Light, Medium, Dark)
Proactiv Solution BB Broad Spectrum SPF 15 (Light, Medium, Dark) by
Drug Labeling and Warnings
Proactiv Solution BB Broad Spectrum SPF 15 (Light, Medium, Dark) by is a Otc medication manufactured, distributed, or labeled by Guthy-Renker LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PROACTIV SOLUTION BB BROAD SPECTRUM SPF 15 (LIGHT, MEDIUM, DARK)- octinoxate, octisalate, and oxybenzone lotion
THE PROACTIV COMPANY LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Proactiv Solution BB Cream Broad Spectrum SPF 15 (Light, Medium, Dark)
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- apply liberally 15 minutes before sun exposure
- reapply:
- after 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive ingredients
water, ethylhexyl palmitate, isostearyl neopentanoate, propylene glycol, stearic acid, propylene glycol stearate SE, glycerin, talc, dimethicone, cetyl alcohol, magnesium ascorbyl phosphate, tocopherol, ascorbyl palmitate, sodium hyaluronate, lecithin, xanthan gum, sorbitan stearate, magnesium aluminum silicate, acrylates/C10-30 alkyl acrylate crosspolymer, ammonium polyacrylate, triethanolamine, disodium EDTA, phenoxyethanol, methylparaben, propylparaben, butylparaben, methylisothiazolinone. may contain (+/-) : iron oxides (CI 77491, CI 77492, CI 77499), titanium dioxide (CI 77891)
| PROACTIV SOLUTION BB BROAD SPECTRUM SPF 15 (LIGHT, MEDIUM, DARK)
octinoxate, octisalate, and oxybenzone lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - THE PROACTIV COMPANY LLC (080216357) |