STAHIST AD- chlorcyclizine hydrochloride and pseudoephedrine hydrochloride tablet
STAHIST AD by
Drug Labeling and Warnings
STAHIST AD by is a Otc medication manufactured, distributed, or labeled by Magna Pharmaceuticals, Inc., Nexgen Pharma, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Do not exceed recommended dosage.
Do not use this product
- If you are taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for two weeks after stopping the MAOI drug. If you do not know if your prescription drug contains MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- heart disease
- high blood pressure
- thyroid disease
- diabetes melitus
- difficulty in urination due to enlargement of the prostate gland
When using this product
- excitability may occur, especially in children
- may cause drowsiness
- alcohol, sedatives and tranquilizers may increase drowsiness effect
- avoid alcoholic beverages
- use caution when driving a motor vehicle or operating machinery
-
Directions
Do not exceed recommended dosage.
Adults and children 12 years of age and over: 1 tablet by mouth every 6-8 hours, not to exceed 3 tablets in 24 hours, or as directed by a doctor Children 6 to under 12 years of age: ½ tablet by mouth every 6-8 hours, not to exceed 1½ tablets in 24 hours, or as directed by a doctor Children under 6 years of age Consult a doctor - Inactive ingredients
- Questions or Comments?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 25 mg/60 mg Tablet Carton
NDC: 58407-625-30
Antihistamine Nasal Decongestant
Stahist AD
Each tablet contains:
Chlorcyclizine HCl 25 mg
Pseudoephedrine HCl 60 mgMAGNA
Pharmaceuticals, Inc.
Louisville, KY 4029930 Tablets
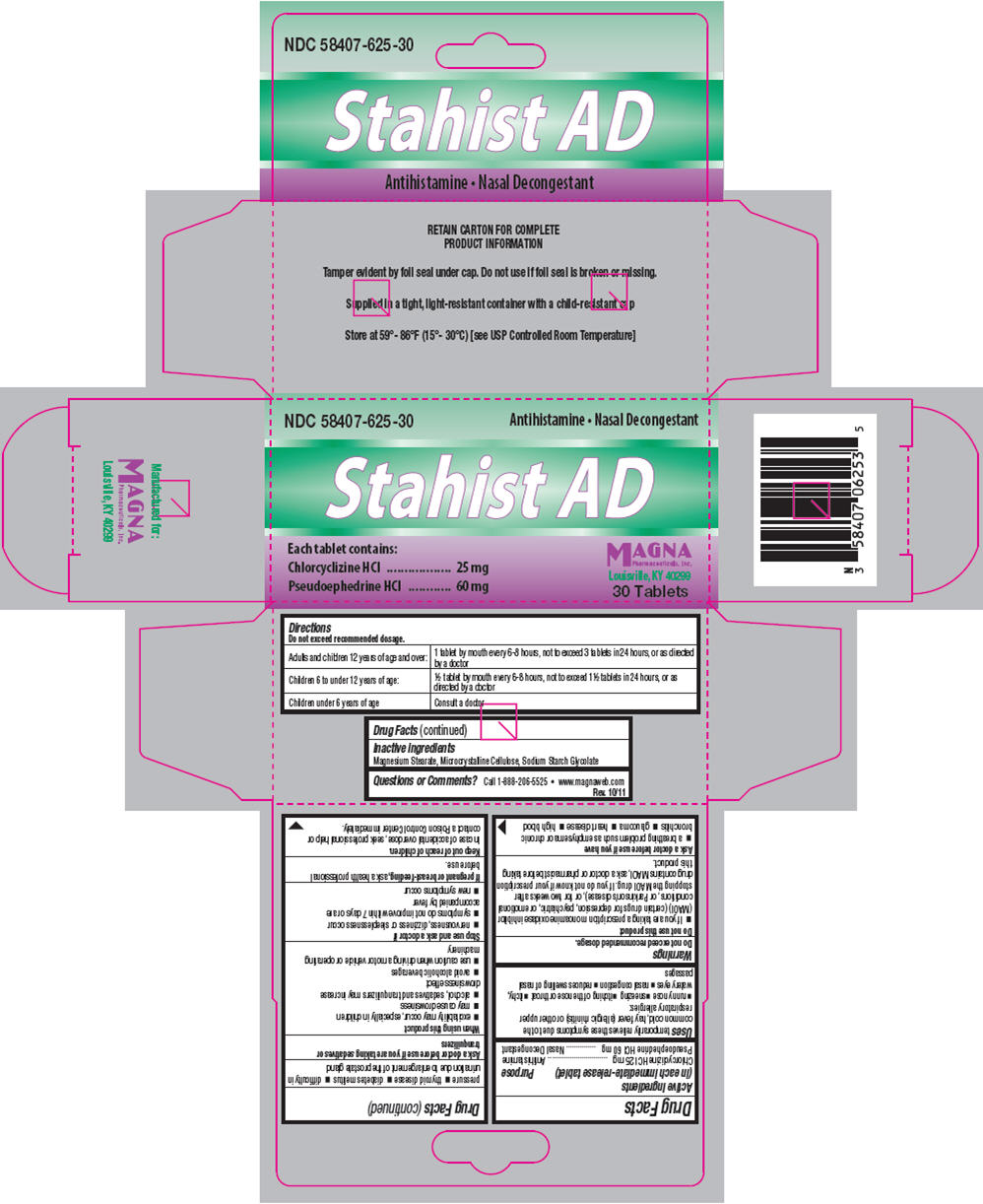
- Sample Package Label
-
INGREDIENTS AND APPEARANCE
STAHIST AD
chlorcyclizine hydrochloride and pseudoephedrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58407-625 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORCYCLIZINE HYDROCHLORIDE (UNII: NPB7A7874U) (CHLORCYCLIZINE - UNII:M26C4IP44P) CHLORCYCLIZINE HYDROCHLORIDE 25 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color white Score 2 pieces Shape OVAL Size 16mm Flavor Imprint Code M625 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58407-625-30 1 in 1 BOX 12/20/2011 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 58407-625-06 6 in 1 BOX 12/20/2011 2 NDC: 58407-625-01 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 58407-625-01 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 09/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 12/20/2011 Labeler - Magna Pharmaceuticals, Inc. (620988360) Establishment Name Address ID/FEI Business Operations Nexgen Pharma, Inc 160356114 manufacture(58407-625)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
