OLOPATADINE- olopatadine hydrochloride solution/ drops. Drug Facts
Olopatadine by
Drug Labeling and Warnings
Olopatadine by is a Otc medication manufactured, distributed, or labeled by Golden State Medical Supply, Inc., Akorn. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
OLOPATADINE- olopatadine hydrochloride solution/ drops
Golden State Medical Supply, Inc.
----------
OLOPATADINE- olopatadine hydrochloride solution/ drops.
Drug Facts
Warnings
For external use only
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
When using the product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before use
- wait at least 10 minutes before reinserting contact lenses after use
- do not wear a contact lens if your eye is red
Directions
-
adults and children 2 years of age and older:
- put 1 drop in the affected eye(s) twice daily, every 6 to 8 hours, no more than twice per day
- if using other ophthalmic products while using this product, wait at least 5 minutes between each product
- replace cap after each use
- children under 2 years of age: consult a doctor
Inactive ingredients
benzalkonium chloride 0.01%, dibasic sodium phosphate, hydrochloric acid and/or sodium hydroxide (adjust pH), sodium chloride, and water for injection
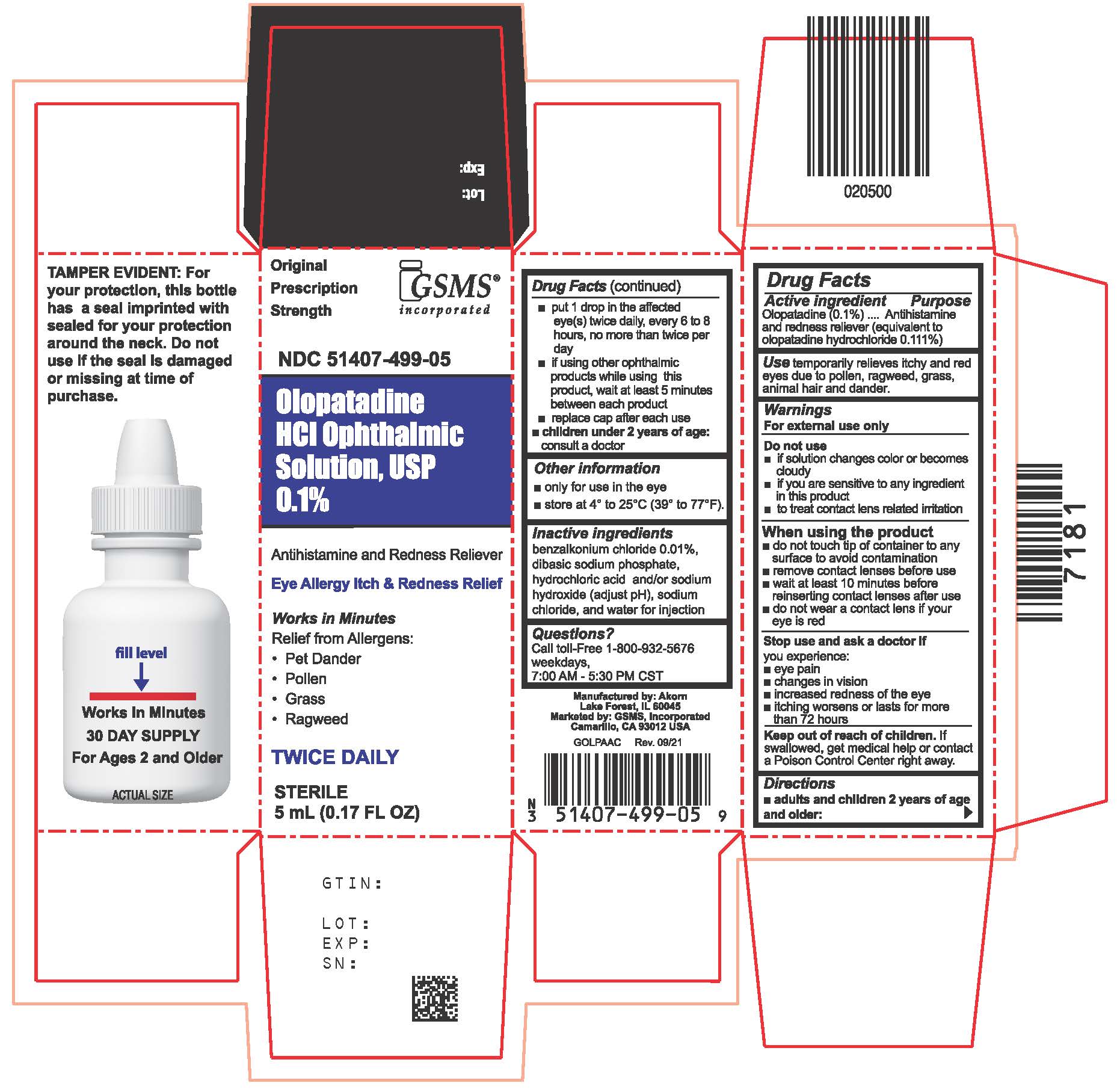
Principal Display Panel Text for Container Label:
NDC: 51407-499-05
Olopatadine HCl Ophthalmic
Solution, USP 0.1%
Antihistamine and Redness Reliever
Eye Allergy Itch & Redness Relief
STERILE 5 mL (0.17 FL OZ)

Principal Display Panel Text for Carton Label:
NDC: 51407-499-05
Prescription
Strength
Olopatadine
HCl Ophthalmic
Solution, USP
0.1%
Antihistamine and Redness Reliever
Eye Allergy Itch & Redness Relief
Works in Minutes
Relief from Allergens:
Pet Dander
Pollen
Grass
Ragweed
TWICE DAILY
STERILE
5 mL (0.17 FL OZ)
| OLOPATADINE
olopatadine hydrochloride solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Golden State Medical Supply, Inc. (603184490) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Akorn | 117696840 | label(51407-499) , manufacture(51407-499) , pack(51407-499) , analysis(51407-499) , sterilize(51407-499) | |