DECIEM The Ordinary Mineral UV Filters SPF30 with Antioxidants
The Ordinary Suncare Mineral UV Filters with Antioxidants by
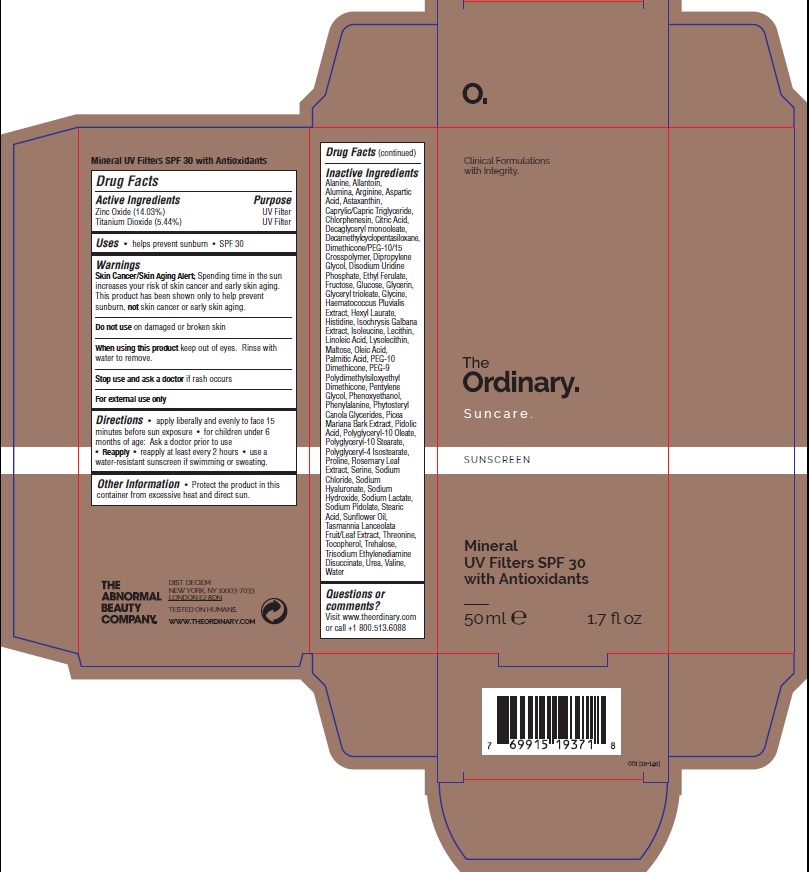
Drug Labeling and Warnings
The Ordinary Suncare Mineral UV Filters with Antioxidants by is a Otc medication manufactured, distributed, or labeled by Deciem Inc, Crystal Claire Cosmetics Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
THE ORDINARY SUNCARE MINERAL UV FILTERS WITH ANTIOXIDANTS SPF30- zinc oxide, titanium dioxid lotionÂ
Deciem Inc
----------
DECIEM The Ordinary Mineral UV Filters SPF30 with Antioxidants
Warnings
​Skin Cancer/Skin Aging Alert; ​Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, ​not​ skin cancer or early skin aging.
Directions
- Â apply liverally and evenly to face 15 minutes before sun exposue
- Â for children under 6 months of age: Ask a doctor prior to use.
- ​Reapply
- ​Reapply at lease every 2 hours
- use a water resistant sunscreen if swimming or sweatingÂ
Other Information
- Protect the product in this container from excessive heat and direct sun
- ​Shake well ​before using
Inactive Ingredients
Water, Cyclopentasiloxane, PEG-10 Dimethicone, Glycerin, Helianthus Annuus Seed Oil, PEG-9 Polydimethylsiloxyethyl Dimethicone, Hexyl Laurate, Polyglyceryl-4 Isostearate, Caprylic/Capric Triglyceride, Dimethicone/PEG-10/15 Crosspolymer, Astaxanthin, Disodium Uridine Phosphate, Ethyl Ferulate, Rosmarinus Officinalis Leaf Extract, Tasmannia Lanceolata Fruit/Leaf Extract, Haematococcus Pluvialis Extract, Picea Mariana Bark Extract, Arginine, Aspartic Acid, Glycine, Alanine, Serine, Valine, Isoleucine, Proline, Threonine, Histidine, Phenylalanine, Sodium PCA, PCA, Sodium Lactate, Glucose, Maltose, Fructose, Trehalose, Urea, Allantoin, Sodium Hyaluronate, Linoleic Acid, Oleic Acid, Phytosteryl Canola Glycerides, Palmitic Acid, Stearic Acid, Isochrysis Galbana Extract, Lysolecithin, Lecithin, Triolein, Pentylene Glycol, Dipropylene Glycol, Polyglyceryl-10 Oleate, Polyglyceryl-5 Trioleate, Polyglyceryl-10 Stearate, Tocopherol, Alumina, Citric Acid, Trisodium Ethylenediamine Disuccinate, Sodium Chloride, Sodium Hydroxide, Phenoxyethanol, Chlorphenesin.
| THE ORDINARY SUNCARE MINERAL UV FILTERS WITH ANTIOXIDANTSÂ
SPF30
zinc oxide, titanium dioxid lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler -Â Deciem Inc (203133665) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.