SIL-Brx PAD by Blaine Labs Inc. SIL-BRX PAD
SIL-Brx PAD by
Drug Labeling and Warnings
SIL-Brx PAD by is a Other medication manufactured, distributed, or labeled by Blaine Labs Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SIL-BRX PAD LARGE- elastomer, silicone, for scar management
Blaine Labs Inc.
----------
SIL-BRX PAD
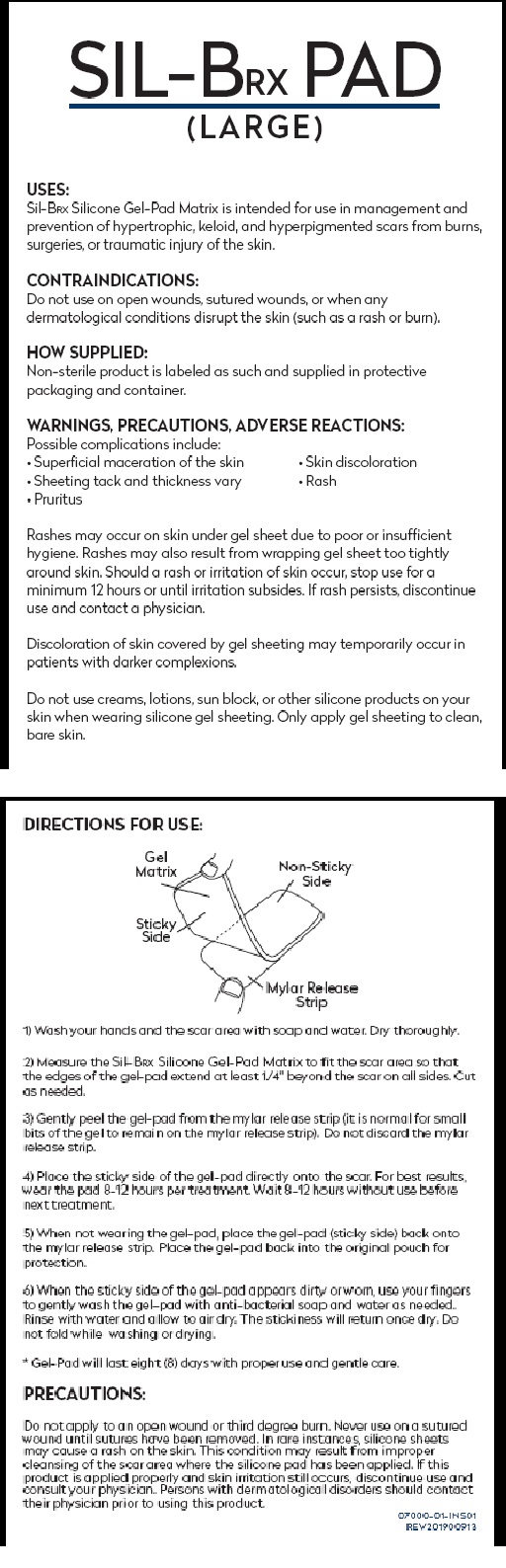
USES:
Sil-BRX Silicone Gel-Pad Matrix is intended for use in management and
prevention of hypertrophic, keloid, and hyperpigmented scars from burns,
surgeries, or traumatic injury of the skin.
CONTRAINDICATIONS:
Do not use on open wounds, sutured wounds, or when any
dermatological conditions disrupt the skin (such as a rash or burn).
HOW SUPPLIED:
Non-sterile product is labeled as such and supplied in protective
packaging and container.
WARNINGS, PRECAUTIONS, ADVERSE REACTIONS:
Possible complications include:
Superficial maceration of the skin Skin discoloration
Sheeting tack and thickness vary Rash
Pruritus
Rashes may occur on skin under gel sheet due to poor or insufficient
hygiene. Rashes may also result from wrapping gel sheet too tightly
around skin. Should a rash or irritation of skin occur, stop use for a
minimum 12 hours or until irritation subsides. If rash persists, discontinue
use and contact a physician.
Discoloration of skin covered by gel sheeting may temporarily occur in
patients with darker complexions.
Do not use creams, lotions, sun block, or other silicone products on your
skin when wearing silicone gel sheeting. Only apply gel sheeting to clean,
bare skin.
DIRECTIONS FOR USE:
1) Wash your hands and the scar area with soap and water. Dry thoroughly.
2) Measure the SIL-BRX PAD to fit the scar area, so that the edges of the gelpad
extend at least 1/4” beyond the scar on all sides. Cut as needed.
3) Gently peel the gel-matrix pad from the mylar release strip (it is normal for
small bits of the gel to remain on the mylar release strip). Do not discard the
mylar release strip.
4) Place the sticky side of the gel-pad directly onto the scar. For best results,
wear the pad 8-12 hours per treatment. Wait 8-12 hours without use before
next treatment.
5) When not wearing the gel-pad, place the gel-pad (sticky side) back onto the
mylar release strip. Place the gel-pad back into the original pouch for
protection.
6) When the sticky side of the gel-pad appears dirty or worn, use your finger to
gently wash the gel-pad with anti-bacterial soap and water, as needed. Rinse with
water and allow to air dry. The stickiness will return once dry. Do not fold while
washing or drying.
* Gel-Pad will last seven (7) days with proper use and gentle care.
PRECAUTIONS:
Do not apply to an open wound or third degree burn. Never use on a sutured
wound until sutures have been removed. In rare instances, silicone sheets
may cause a rash on the skin. This condition may result from improper
cleansing of the scar area where the silicone pad has been applied. If this
product is applied properly and skin irritation still occurs, discontinue use and
consult your physician. Persons with dermatological disorders should contact
their physician prior to using this product.
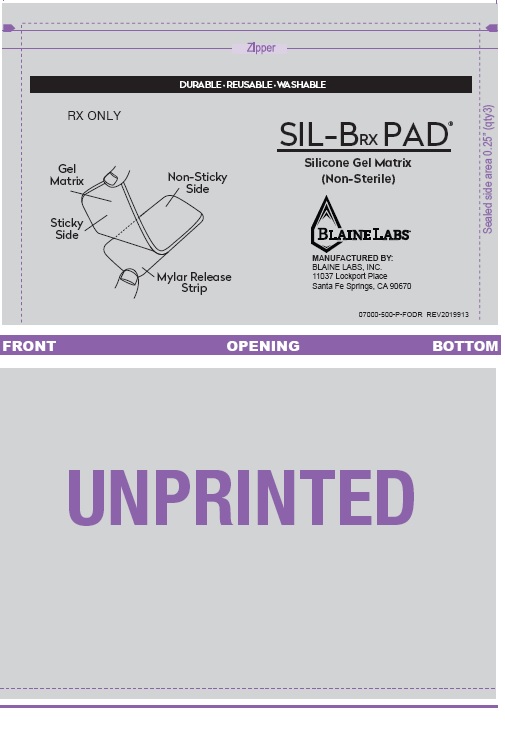
Package Labeling
RX ONLY
BLAINE LABS®
NDC# 63347-160-01
MDA-K001608
US PATENT # 6,572,878
SIL-BRX PAD
(LARGE)
BY PRESCRIPTION ONLY
BLAINE LABS®
SIL-BRX PAD
(LARGE)
LOT NO.
MEDICAL-GRADE SILICONE GEL MATRIX
PATENTED FORMULA
FOUR (4) GEL-PADS - 2” x 5.5” (NON-STERILE)
RX ONLY
BLAINE LABS®
MANUFACTURED BY:
BLAINE LABS, INC.
11037 LOCKPORT PLACE
SANTA FE SPRINGS, CA 90670
MADE IN THE USA
THIS PACKAGE IS MADE FROM
RECYCLABLE MATERIALS.
07000-500-BX
REV201900913

RES
| SIL-BRX PAD
LARGE
elastomer, silicone, for scar management |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Blaine Labs Inc. (017314571) |
| Registrant - Blaine Labs Inc. (017314571) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Blaine Labs Inc. | 017314571 | manufacture | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.