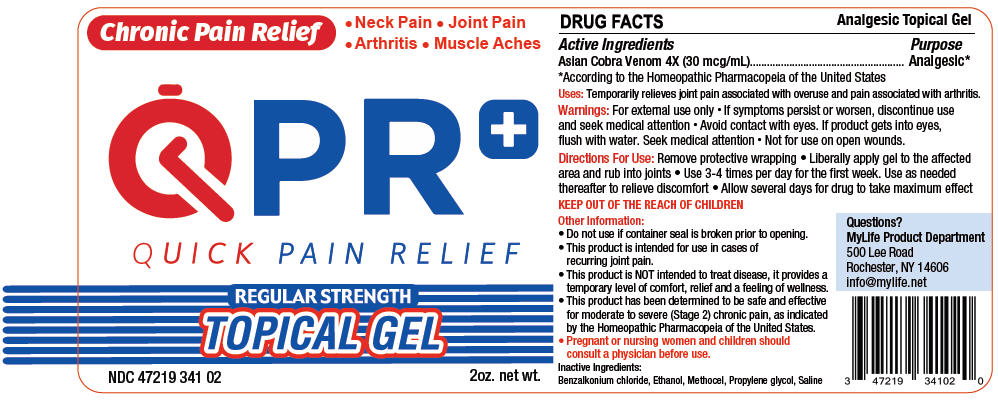
QPR QUICK PAIN RELIEF- naja naja venom gel
QPR Quick Pain Relief by
Drug Labeling and Warnings
QPR Quick Pain Relief by is a Homeopathic medication manufactured, distributed, or labeled by Nutra Pharma Corporation, Receptopharm, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions For Use
- KEEP OUT OF REACH OF CHILDREN
-
Other Information
- Do not use if container seal is broken prior to opening.
- This product is intended for use in cases of recurring joint pain.
- This product is NOT intended to treat disease, it provides a temporary level of comfort, relief and a feeling of wellness.
- This product has been determined to be safe and effective for moderate to severe (Stage 2) chronic pain, as indicated by the Homeopathic Pharmacopeia of the United States.
- Pregnant or nursing women and children should consult a physician before use.
- Inactive Ingredients
- Questions?
- PRINCIPAL DISPLAY PANEL - 60 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
QPR QUICK PAIN RELIEF
naja naja venom gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 47219-341(NDC:47219-301) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Naja Naja Venom (UNII: ZZ4AG7L7VM) (Naja Naja Venom - UNII:ZZ4AG7L7VM) Naja Naja Venom 4 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength Benzalkonium chloride (UNII: F5UM2KM3W7) Alcohol (UNII: 3K9958V90M) Propylene Glycol (UNII: 6DC9Q167V3) Sodium Chloride (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 47219-341-02 60 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 09/15/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 09/15/2016 Labeler - Nutra Pharma Corporation (141236286) Establishment Name Address ID/FEI Business Operations Receptopharm, Inc 145377888 API MANUFACTURE(47219-341) , REPACK(47219-341) , RELABEL(47219-341)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.