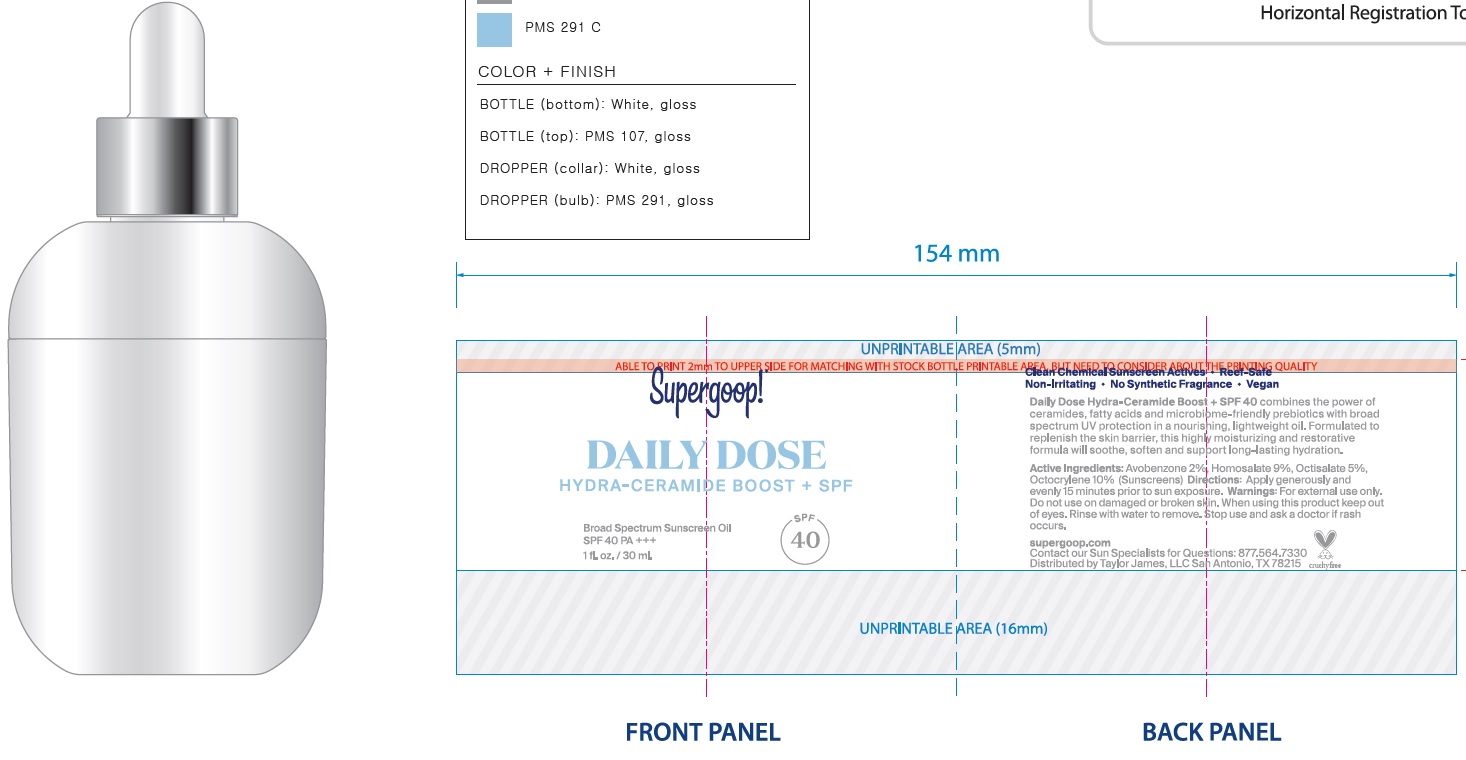
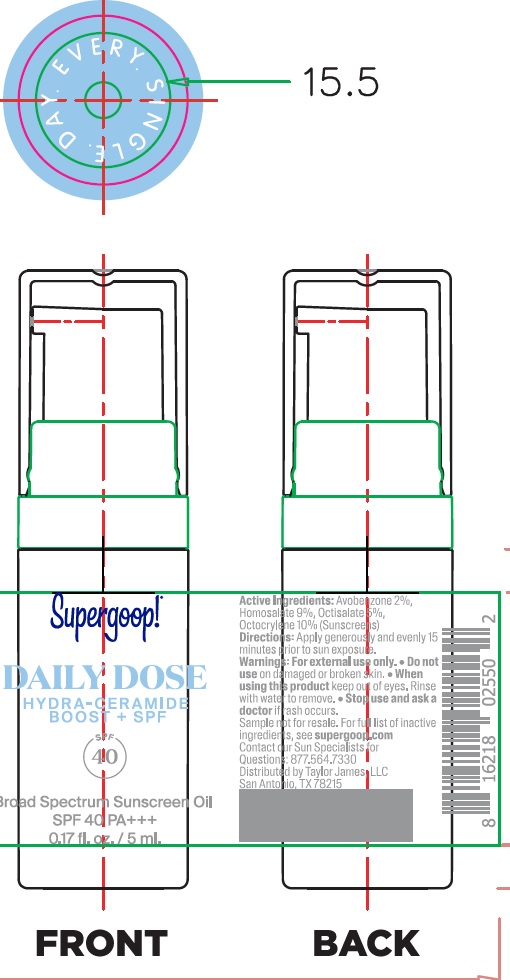
Supergoop! Daily Hydra-Ceramide Boost + SPF40
Supergoop Daily Hydra-Ceramide Boost SPF40 by
Drug Labeling and Warnings
Supergoop Daily Hydra-Ceramide Boost SPF40 by is a Otc medication manufactured, distributed, or labeled by HK Kolmar Canada, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SUPERGOOP DAILY HYDRA-CERAMIDE BOOST SPF40- avobenzone, homosalate, octisalate, octocrylene oil
HK Kolmar Canada, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Supergoop! Daily Hydra-Ceramide Boost + SPF40
Uses
Helps prevent sunburn If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
apply generously and evenly 15 minutes before sun exposure reapply at least every 2 hours use a water resistant sunscreen if swimming or sweating Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m. – 2 p.m. Wear long-sleeved shirts, pants, hats, and sunglasses Children under 6 months: Ask a doctor
Inactive Ingredients
Caprylic/Capric Triglyceride, C12-15 Alkyl Benzoate, Dicaprylyl Ether, Butyloctyl Salicylate, Polybutene, Ethyl Olivate, Rosa Canina Seed Oil, Jojoba Oil/Macadamia Seed Oil Esters, Hydrogenated Polycyclopentadiene, Squalane, Glycine Soja (Soybean) Oil, Simmondsia Chinensis (Jojoba) Seed Oil, Squalene, Polyglyceryl-3 Diisostearate, Tocopherol, Oryza Sativa (Rice) Extract, Oryza Sativa (Rice) Germ Extract, Avena Sativa (Oat) Kernel Oil, Phytosteryl Macadamiate, Ceramide NP (Ceramide III B), Olea Europaea (Olive) Leaf Extract, Bacillus Ferment, Phytosterols, Ricinus Communis (Castor) Seed Oil, Sodium Hyaluronate, Helianthus Annuus (Sunflower) Seed Wax
| SUPERGOOP DAILY HYDRA-CERAMIDE BOOST SPF40
avobenzone, homosalate, octisalate, octocrylene oil |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - HK Kolmar Canada, Inc (243501959) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.