Jupiter Wellness (as PLD) - Photocil (82301-001) - DELIST
Photocil by
Drug Labeling and Warnings
Photocil by is a Otc medication manufactured, distributed, or labeled by Jupiter Wellness, Inc., Health Specialty. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PHOTOCIL- dimethicone cream
Jupiter Wellness, Inc.
----------
Jupiter Wellness (as PLD) - Photocil (82301-001) - DELIST
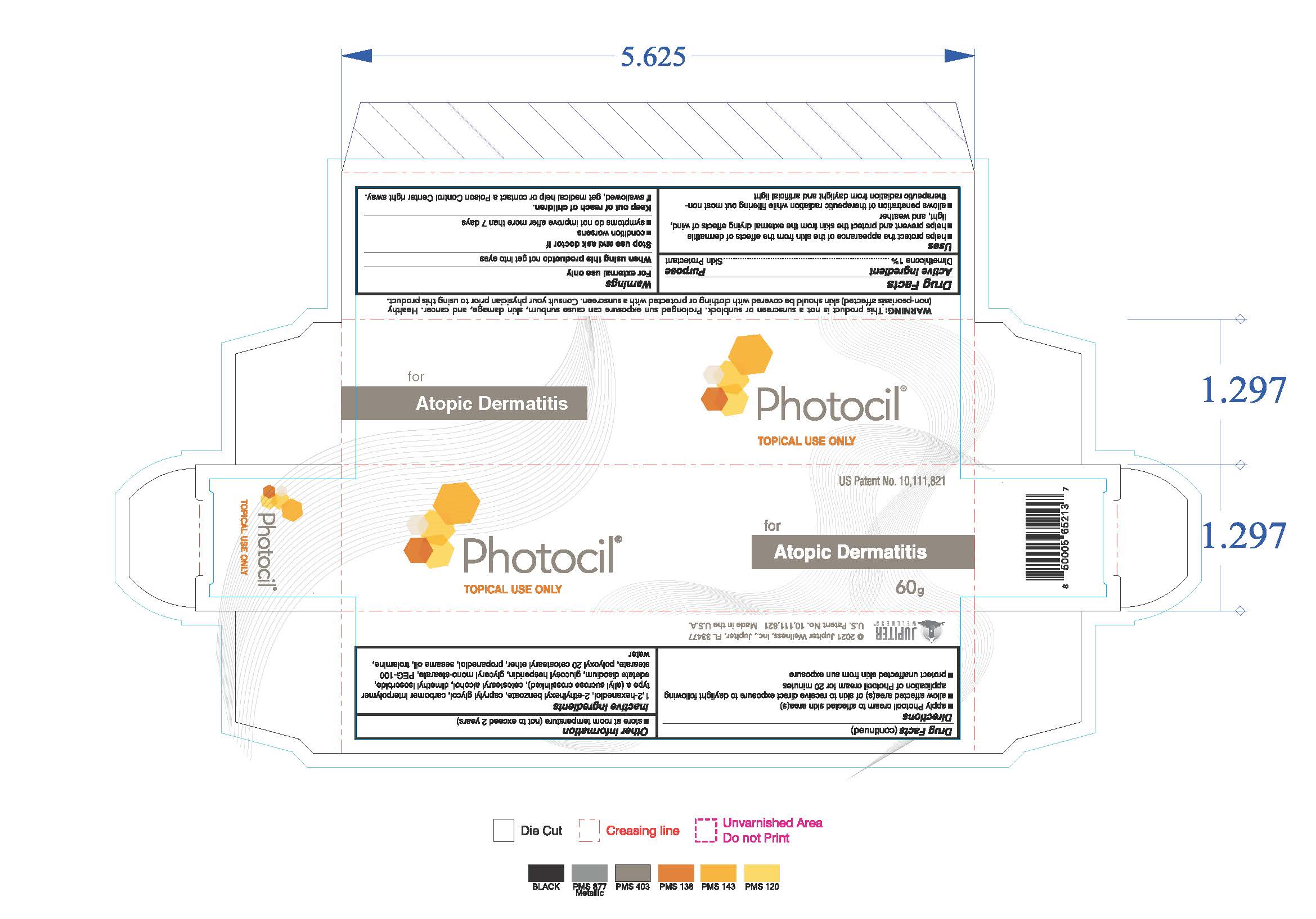
Uses
- helps protect the appearance of the skin from the effects of dermatitis
- helps prevent and protect the skin from the external drying effects of wind, light and weather
- allows penetration of therapeutic radiation while filtering out most non-therapeutic radiation from daylight and artificial light
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Directions
- apply Photocil cream to affected skin area(s)
- allow affected area(s) of skin to receive direct exposure to daylight following application of Photocil cream for 20 minutes
- protect unaffected skin from sun exposure
Inactive ingredients
1,2-hexanediol, 2-ethylhexyl benzoate, caprylyl glycol, carbomer interpolymer type a (allyl sucrose crosslinked), cetostearyl alcohol,
dimethyl isosorbide, edetate disodium, glucosyl hesperidin, glyceryl mono-stearate, PEG-100 stearate, polyoxyl 20 cetostearyl ether,
propanediol, sesame oil, trolamine, water
| PHOTOCIL
dimethicone cream |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Jupiter Wellness, Inc. (118320452) |
Revised: 3/2024
Document Id: 14bc483c-15bf-9ea9-e063-6294a90a80ae
Set id: cd9c8789-b9b9-5840-e053-2995a90a1555
Version: 3
Effective Time: 20240328
Trademark Results [Photocil]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PHOTOCIL 85818859 4851587 Live/Registered |
Applied Biology, Inc. 2013-01-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.