Photocil by Jupiter Wellness, Inc. / Health Specialty Drug Facts
Photocil by
Drug Labeling and Warnings
Photocil by is a Otc medication manufactured, distributed, or labeled by Jupiter Wellness, Inc., Health Specialty. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PHOTOCIL- dimethicone cream
Jupiter Wellness, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
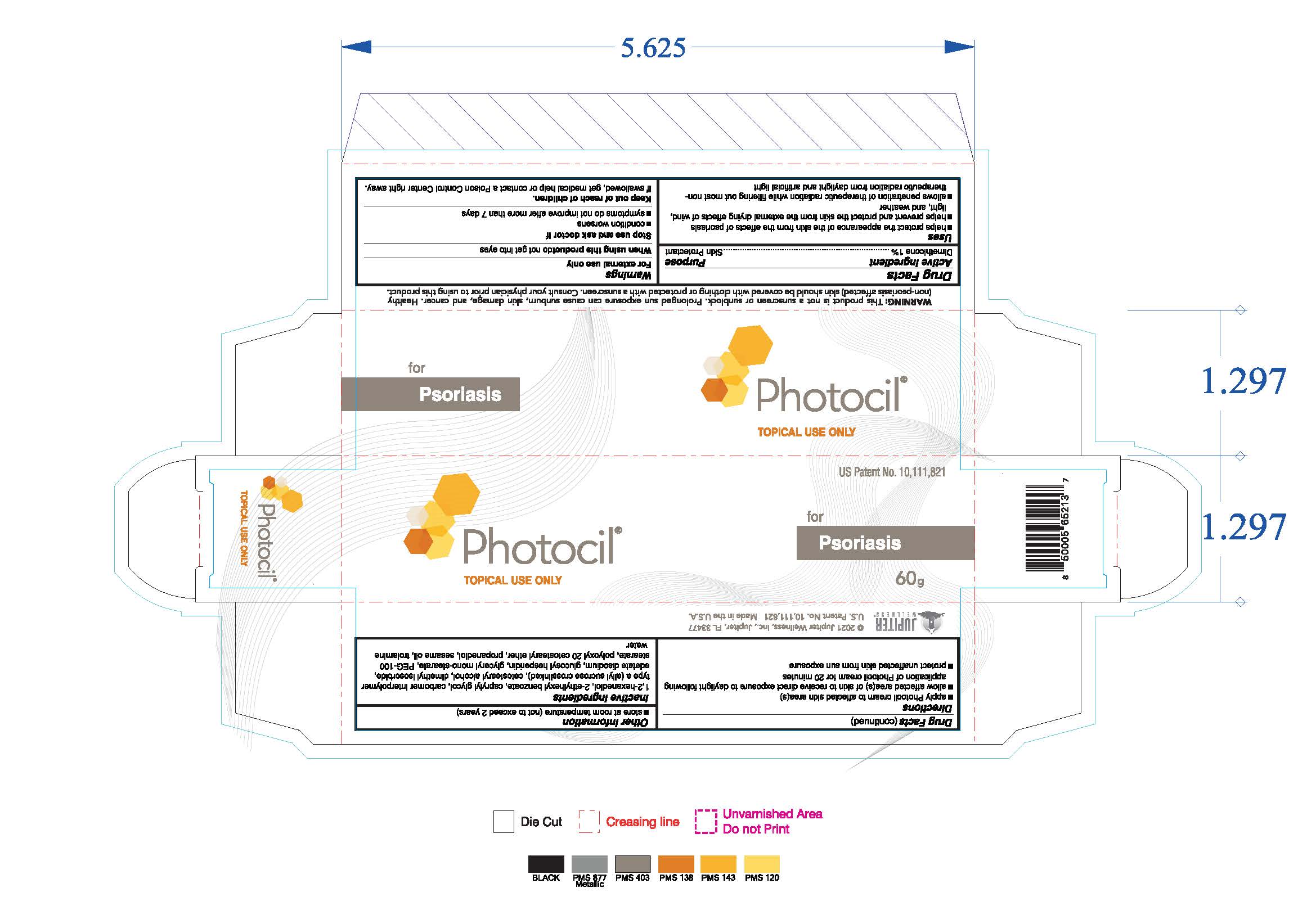
Drug Facts
Uses
- helps protect the appearance of the skin from the effects of psoriasis
- helps prevent and protect the skin from the external drying effects of wind, light and weather
- allows penetration of therapeutic radiation while filtering out most non-therapeutic radiation from daylight and artificial light
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply Photocil cream to affected skin area(s)
- allow affected area(s) of skin to receive exposure to daylight following application of Photocil cream for 20 minutes
- protect unaffected skin from sun exposure
Inactive ingredients
1,2-hexanediol, 2-ethylhexyl benzoate, caprylyl glycol, carbomer interpolymer type a (allyl sucrose crosslinked), cetostearyl alcohol,
dimethyl isosorbide, edetate disodium, glucosyl hesperidin, glyceryl mono-stearate, PEG-100 stearate, polyoxyl 20 cetostearyl ether,
propanediol, sesame oil, trolamine, water
| PHOTOCIL
dimethicone cream |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Jupiter Wellness, Inc. (118320452) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Health Specialty | 794053863 | manufacture(82301-002) | |
Trademark Results [Photocil]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PHOTOCIL 85818859 4851587 Live/Registered |
Applied Biology, Inc. 2013-01-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.