PROCTOFOAM NS- pramoxine hydrochloride aerosol, foam
PROCTOFOAM by
Drug Labeling and Warnings
PROCTOFOAM by is a Otc medication manufactured, distributed, or labeled by Meda Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
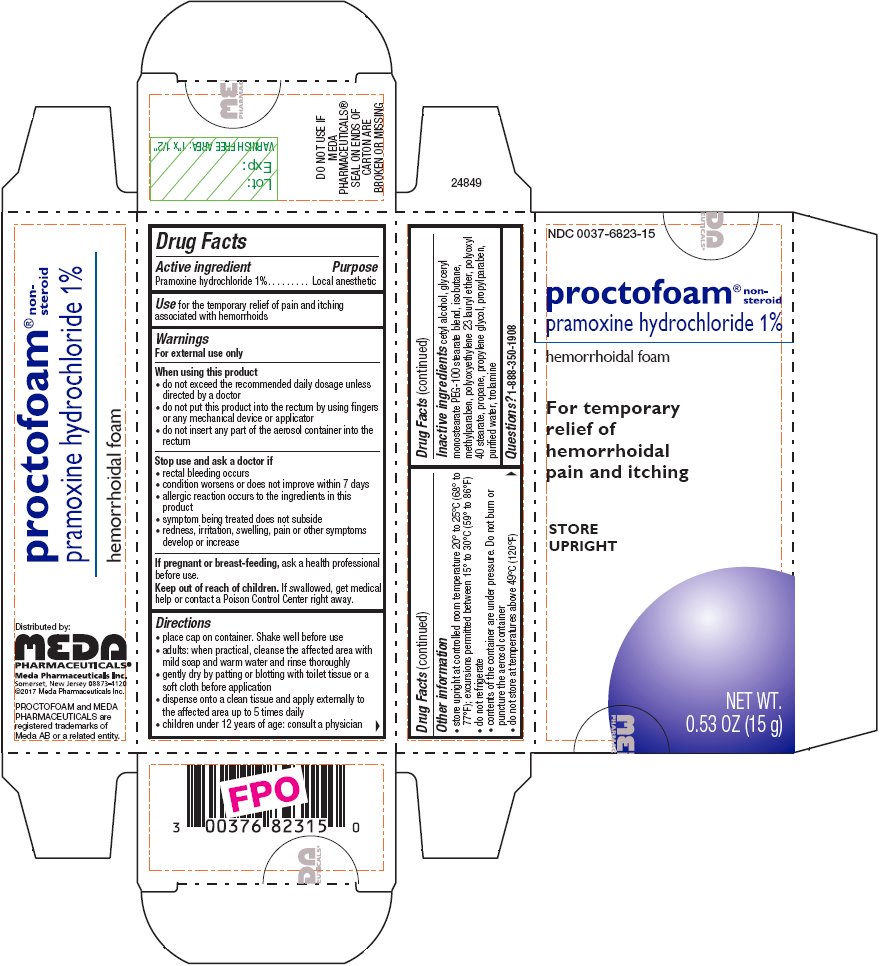
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- do not exceed the recommended daily dosage unless directed by a doctor
- do not put this product into the rectum by using fingers or any mechanical device or applicator
- do not insert any part of the aerosol container into the rectum
-
Directions
- place cap on container. Shake well before use
- adults: when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly
- gently dry by patting or blotting with toilet tissue or a soft cloth before application
- dispense onto a clean tissue and apply externally to the affected area up to 5 times daily
- children under 12 years of age: consult a physician
- Other information
- Inactive ingredients
- Questions?
-
Principal Display Panel – 1%
NDC: 0037-6823-15
proctofoam® non-steroid
pramoxine hydrochloride 1%
hemorrhoidal foamFor temporary
relief of
hemorrhoidal
pain and itchingSTORE
UPRIGHTNET WT.
0.53 OZ (15 g) -
INGREDIENTS AND APPEARANCE
PROCTOFOAM NS
pramoxine hydrochloride aerosol, foamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0037-6823 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 150 mg in 15 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) METHYLPARABEN (UNII: A2I8C7HI9T) LAURETH-23 (UNII: N72LMW566G) POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) ISOBUTANE (UNII: BXR49TP611) PROPANE (UNII: T75W9911L6) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0037-6823-15 1 in 1 CARTON 08/18/2014 1 15 g in 1 CANISTER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 08/18/2014 Labeler - Meda Pharmaceuticals (051229602)
Trademark Results [PROCTOFOAM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PROCTOFOAM 72239316 0819021 Live/Registered |
REED & CARNRICK 1966-02-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.