TriStart FREE by Carwin Pharmaceutical Associates, LLC TriStart™ Free
TriStart FREE by
Drug Labeling and Warnings
TriStart FREE by is a Other medication manufactured, distributed, or labeled by Carwin Pharmaceutical Associates, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TRISTART FREE- ascorbic acid, cholecalciferol, .alpha.-tocopherol, d-, pyridoxine hydrochloride, riboflavin, niacin, folic acid, iron, magnesium oxide, and schizochytrium dha capsule, liquid filled
Carwin Pharmaceutical Associates, LLC
----------
TriStart™ Free
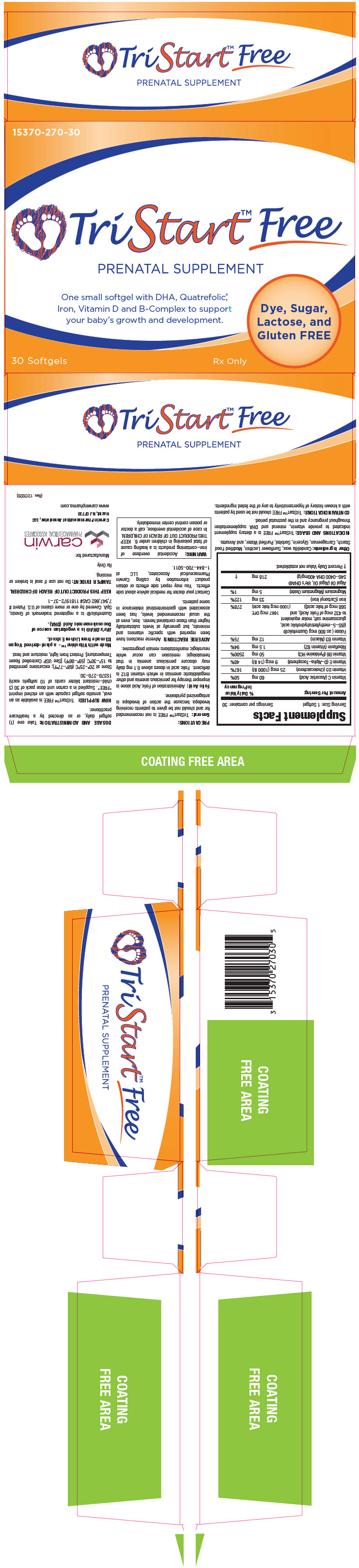
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 Softgel | Servings per container: 30 | |
| Amount Per Serving: | % Daily Value In Pregnancy |
|
|
|
||
| Vitamin C (Ascorbic Acid) | 60 mg | 50% |
| Vitamin D3 (Cholecalciferol) | 25 mcg (1000 IU) | 167% |
| Vitamin E (D–Alpha–Tocopherol) | 9 mcg (14 IU) | 48% |
| Vitamin B6 (Pyridoxine HCl) | 50 mg | 2500% |
| Riboflavin (Vitamin B2) | 1.5 mg | 94% |
| Vitamin B3 (Niacin) | 12 mg | 75% |
| Folate ( as 800 mcg Quatrefolic® ((6S–5–methyltetrahydrofolic acid, glucosamine salt, molar equivalent to 432 mcg of Folic Acid), and 568 mcg of folic acid)) | 1667 mcg DFE (1000 mcg folic acid) | 278% |
| Iron (Carbonyl Iron) | 33 mg | 122% |
| Magnesium (Magnesium Oxide) | 5 mg | 1% |
| Algal Oil (Algal Oil, life's DHA® S40–0400 DHA 400mg/g) | 218 mg | * |
Other Ingredients: Candelilla wax, Sunflower Lecithin, Modified Food Starch, Carrageenan, Glycerin, Sorbitol, Purified Water, and Annatto.
INDICATIONS AND USAGE
TriStart™ FREE is a dietary supplement indicated to provide vitamin, mineral and DHA supplementation throughout pregnancy and in the postnatal period.
CONTRAINDICATIONS
TriStart™ FREE should not be used by patients with a known history of hypersensitivity to any of the listed ingredients.
PRECAUTIONS
General
TriStart™ FREE is not recommended for and should not be given to patients receiving levodopa because the action of levodopa is antagonized pyridoxine.
Folic Acid
Administration of Folic Acid alone is improper therapy for pernicious anemia and other megaloblastic anemias in which vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive.
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals, but generally at levels substantially higher than those contained herein. Iron, even at the usual recommended levels, has been associated with gastrointestinal intolerance in some patients.
Contact your doctor for medical advice about side effects. You may report side effects or obtain product information by calling Carwin Pharmaceutical Associates, LLC at 1–844–700–5011.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
HOW SUPPLIED
TriStart™ FREE is available as an oval, annatto softgel capsule with an etched imprint "FREE". Supplied in a carton unit dose pack of 30 (3 child-resistant blister cards of 10 softgels each) 15370–270–30.
Store at 20°–25°C (68°–77°F); excursions permitted to 15°–30°C (59°–86°F) [See USP Controlled Room Temperature]. Protect from light, moisture and heat.
Made with Vitashine™ - a plant-derived Vegan D3 made from Lichen Extract.
life's DHA® is a vegetarian source of Docosahexaenoic Acid (DHA).
Quatrefolic® is a registered trademark of Gnosis, SpA. Covered by one or more claims of U.S. Patent # 7,947,662 CAS# 1181972–37–1
KEEP THIS PRODUCT OUT OF REACH OF CHILDREN.
TAMPER EVIDENT: Do not use if seal is broken or missing.
Dispensed by a Prescription
Manufactured for:
Carwin Pharmaceutical Associates, LLC
Hazlet, NJ 07730
www.carwinpharma.com
(Rev. 12/2020)
PRINCIPAL DISPLAY PANEL - 30 Softgel Blister Pack Carton
15370-270-30
TriStart™ Free
PRENATAL SUPPLEMENT
One small softgel with DHA, Quatrefolic®,
Iron, Vitamin D and B Vitamins to support
your baby's growth and development.
Dye, Sugar,
Lactose, and
Gluten FREE
30 Softgels
Dispensed by a Prescription
| TRISTART FREE
ascorbic acid, cholecalciferol, .alpha.-tocopherol, d-, pyridoxine hydrochloride, riboflavin, niacin, folic acid, iron, magnesium oxide, and schizochytrium dha capsule, liquid filled |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 12 mm | |
| imprint | ||
| Labeler - Carwin Pharmaceutical Associates, LLC (079217215) |