SC HERBAL PAIN RELIEVING
SC HERBAL PAIN RELIEVING by
Drug Labeling and Warnings
SC HERBAL PAIN RELIEVING by is a Otc medication manufactured, distributed, or labeled by Big 5 Nutrition LLC, TAIWAN SHUENN-AN BIOTECHNOLOGY PHARMACEUTICAL CO., LTD.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SC HERBAL PAIN RELIEVING- menthol, methyl salicylate patch
Big 5 Nutrition LLC
----------
SC HERBAL PAIN RELIEVING
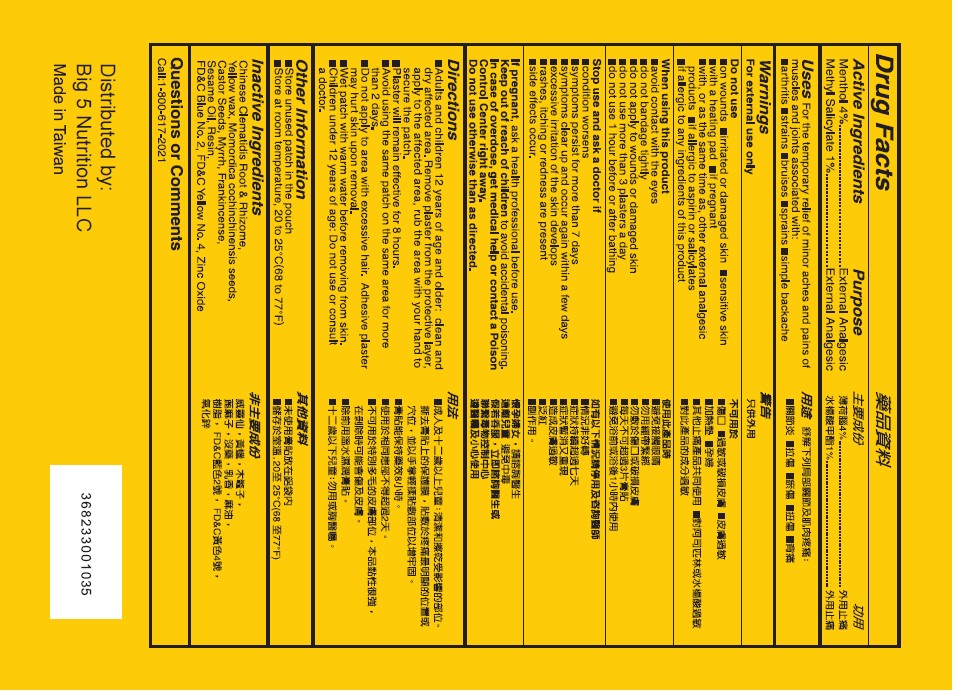
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with: ■ arthritis ■ strains ■ bruises ■ sprains
■ simple backache
Warnings
For external use only
Do not use
■on wounds ■ irritated or damaged skin ■ sensitive skin ■ with a heating pad ■ if pregnant ■ with, or as the same time as, other external analgesic products ■ if allergic to aspirin or salicylates ■ if allergic to any ingredients of this product
When using this product
■ avoid contact wit the eyes
■ do not bandage tightly
■ do not apply to wounds or damaged skin
■ do not use more than 3 plasters a day
■ do not use 1 hour before or after bathing
Directions
■ Adults and children 12 years of age and older: clean and dry affected area. Remove patch from the protective layer, apply to the affected area, rub the area with your hand to secure the patch.
■ Patch will remain effective for 8 hours.
■ Avoid using the same patch on the same area for more than 2 days.
■ Do not apply to area with excessive hair. Adhesive patch may hurt skin upon removal.
■ Wet patch with warm water before removing from skin
■ Children under 12 years of age: Do not use or consult a doctor.
Other information
■ store unused patch in the pouch
■ store at room temperature, 20° to 25° C(68° to 77° F
| SC HERBAL PAIN RELIEVING
menthol, methyl salicylate patch |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Big 5 Nutrition LLC (114574559) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| TAIWAN SHUENN-AN BIOTECHNOLOGY PHARMACEUTICAL CO., LTD. | 656348265 | manufacture(82198-0003) , pack(82198-0003) , label(82198-0003) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.