ARNICARE- arnica montana gel
Arnicare by
Drug Labeling and Warnings
Arnicare by is a Homeopathic medication manufactured, distributed, or labeled by Laboratoires Boiron, Boiron Inc., Boiron. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
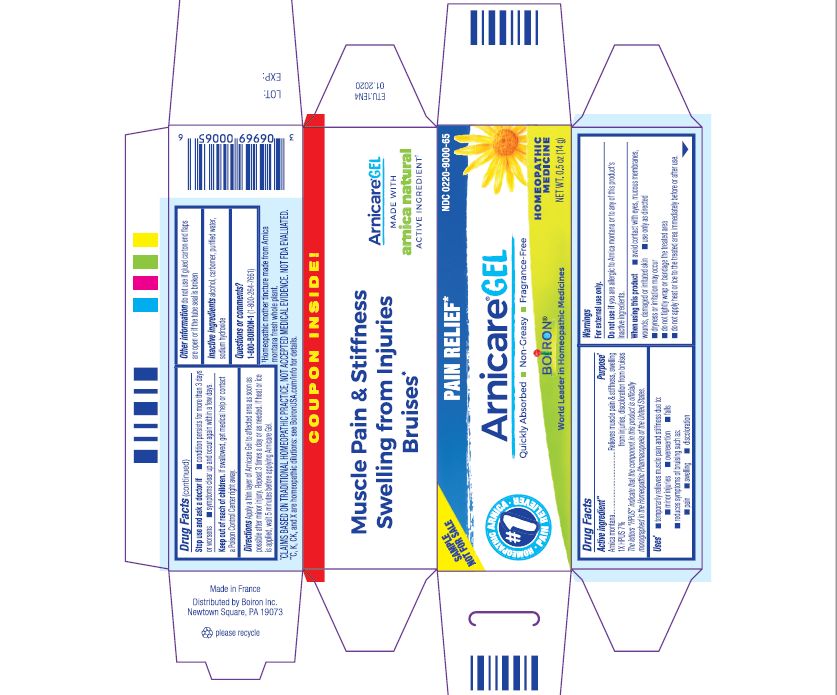
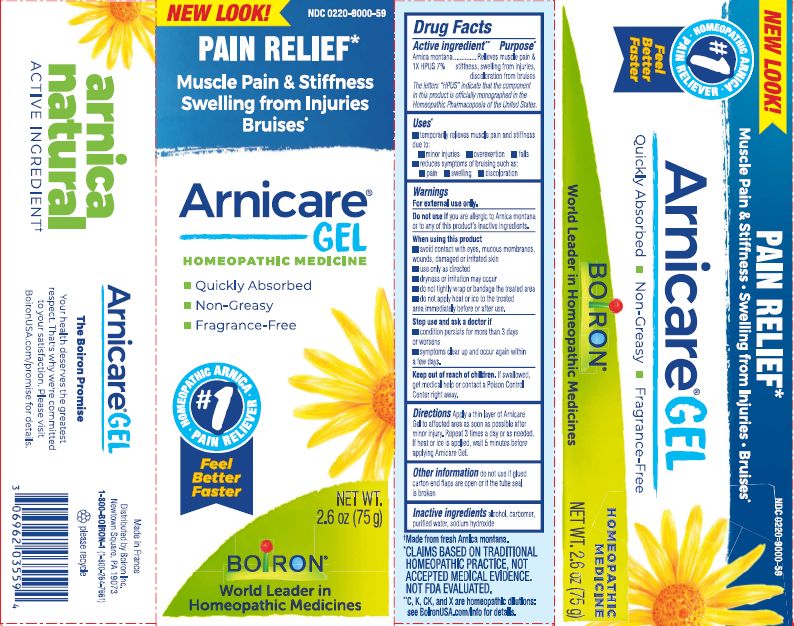
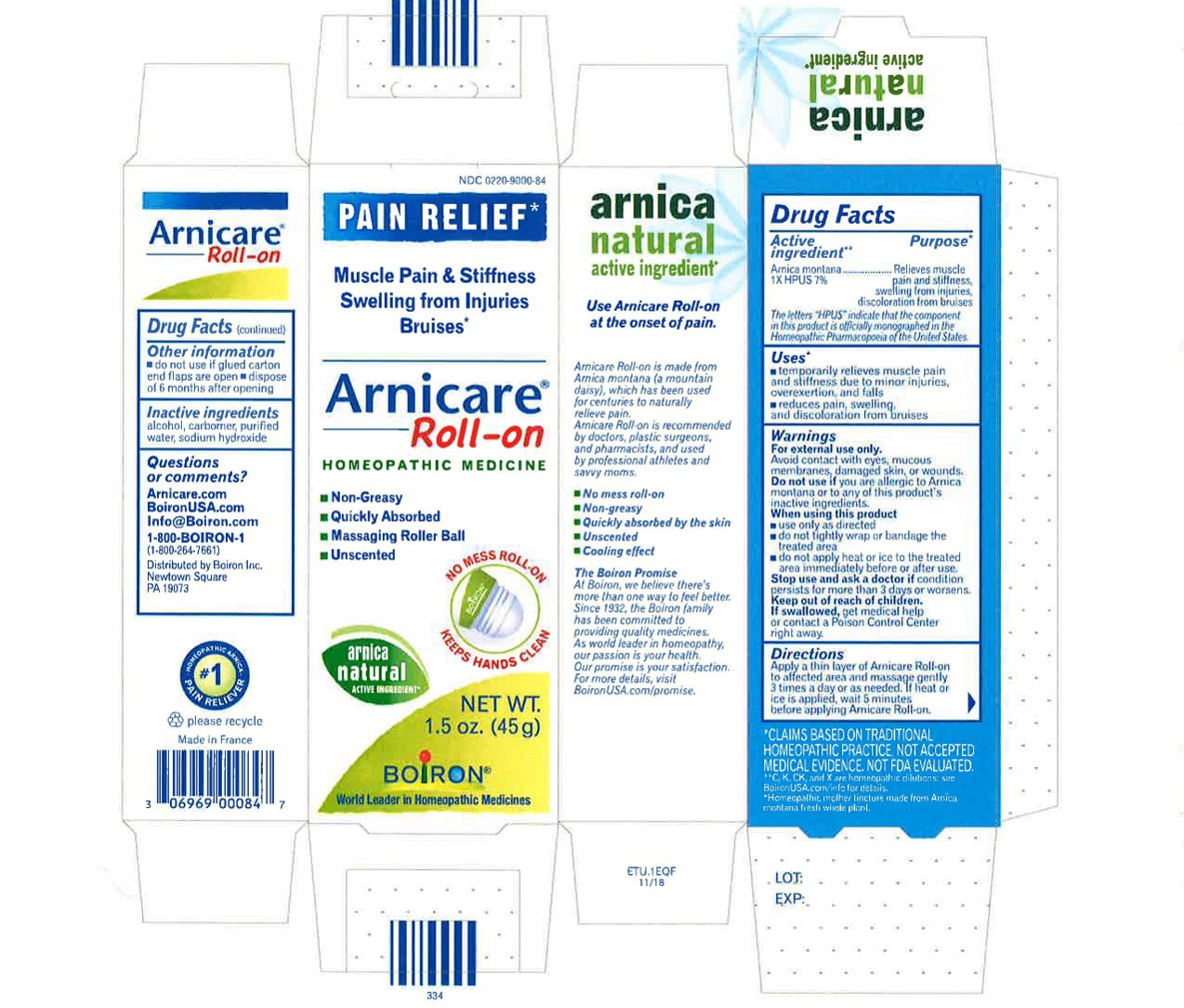
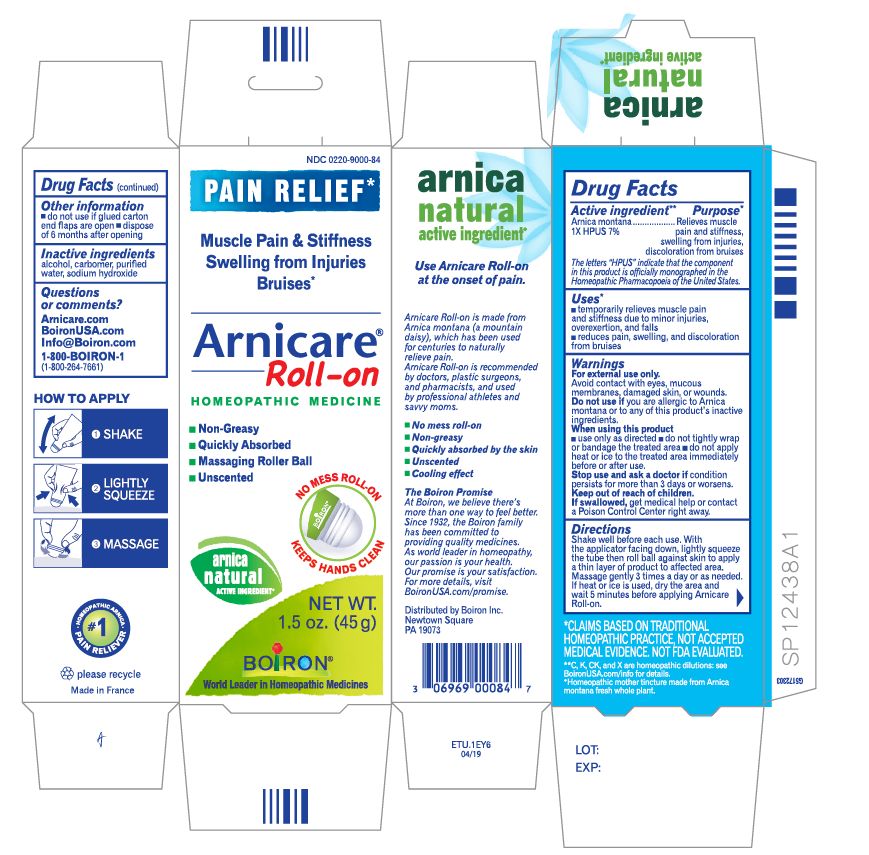
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For external use only
When using this product
Avoid contact with eyes, mucous membranes, wounds, damaged or irritated skin.Do not use if you are allergic to Arnica montana or to any of this product's inactive ingredients.
Use only as directed
dryness or irritation may occur
do not tightly wrap or bandage the treated area
do not apply heat or ice to treated area immediately before or after use.
- DO NOT USE
- ASK DOCTOR
- STOP USE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- HOW SUPPLIED
- STORAGE AND HANDLING
- QUESTIONS
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- WHEN USING
- DRUG INTERACTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ARNICARE
arnica montana gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0220-9000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0220-9000-66 1 in 1 PACKAGE 01/09/2007 1 120 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 0220-9000-59 1 in 1 PACKAGE 01/09/2007 2 75 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC: 0220-9000-54 1 in 1 PACKAGE 01/09/2007 3 45 g in 1 TUBE; Type 0: Not a Combination Product 4 NDC: 0220-9000-65 1 in 1 PACKAGE 01/09/2007 4 14 g in 1 TUBE; Type 0: Not a Combination Product 5 NDC: 0220-9000-84 1 in 1 PACKAGE 06/01/2018 5 45 g in 1 TUBE; Type 0: Not a Combination Product 6 NDC: 0220-9000-86 2 in 1 PACKAGE 06/01/2018 6 45 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/09/2007 Labeler - Laboratoires Boiron (282560473) Registrant - Boiron Inc. (014892269) Establishment Name Address ID/FEI Business Operations Boiron 282560473 manufacture(0220-9000)
Trademark Results [Arnicare]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ARNICARE 97591041 not registered Live/Pending |
BOIRON 2022-09-14 |
 ARNICARE 88258237 5941835 Live/Registered |
BOIRON 2019-01-11 |
 ARNICARE 76312567 2749409 Live/Registered |
BOIRON 2001-09-14 |
 ARNICARE 74330888 1841163 Dead/Cancelled |
LABORATORIES DOLISOS 1992-11-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.