GENOZOL- esomeprazole magnesium capsule, delayed release
GENOZOL by
Drug Labeling and Warnings
GENOZOL by is a Otc medication manufactured, distributed, or labeled by Genomma Lab USA Inc, Aurohealth LLC, Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
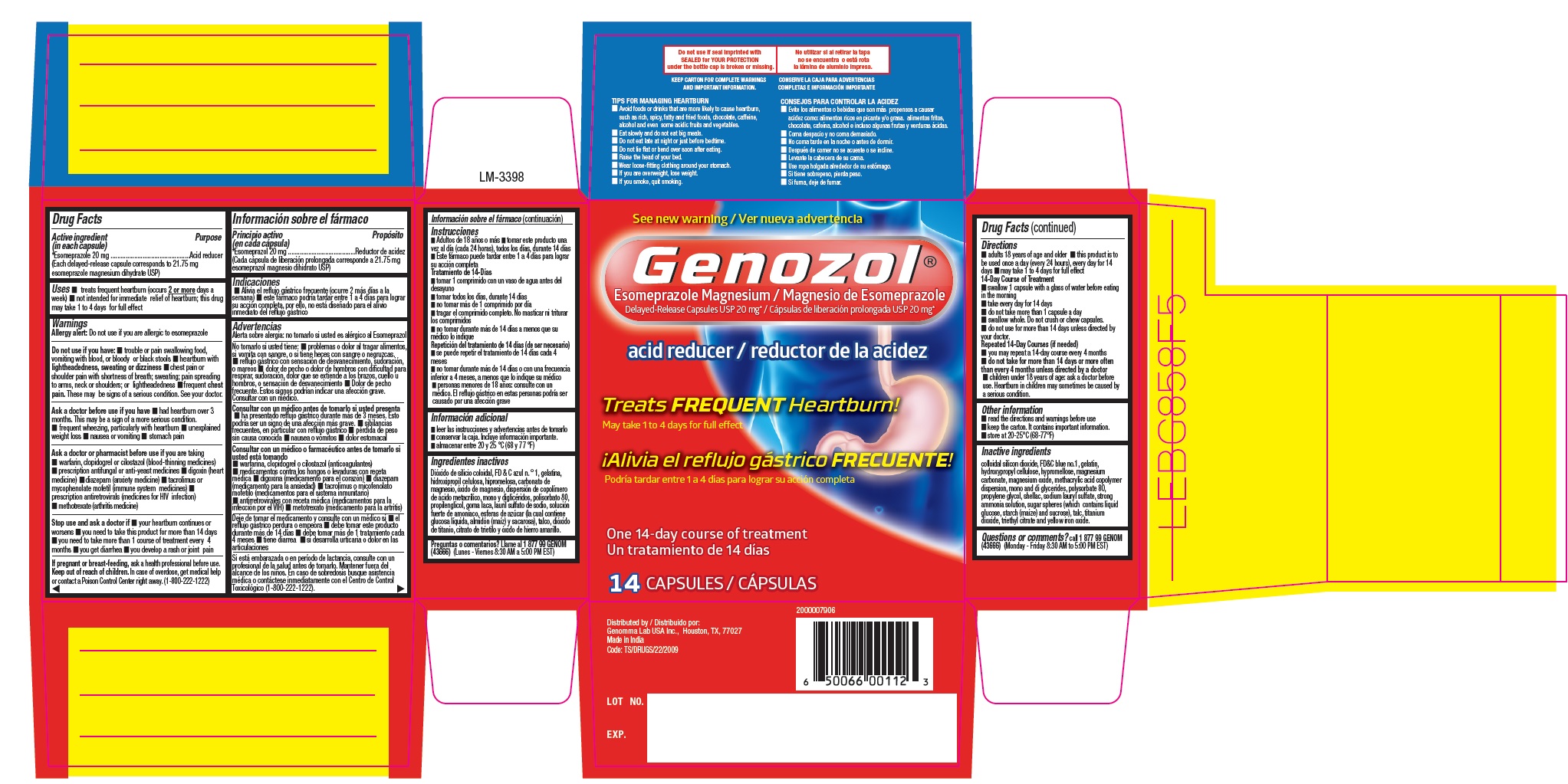
- Drug Facts
- Purpose
- Uses
- Warnings
-
Do not use if you have:
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- heartburn with lightheadedness, sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
These may be signs of a serious condition. See your doctor.
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- may take 1 to 4 days for full effect
14-Day Course of Treatment
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules.
- do not use for more than 14 days unless directed by your doctor
Repeated 14-Day Courses (if needed)
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
Other information
- read the directions and warnings before use
- keep the carton. It contains important information.
- store at 20-25°C (68-77°F)
-
Inactive ingredients
colloidal silicon dioxide, FD&C blue no.1, gelatin, hydroxypropyl cellulose, hypromellose, magnesium carbonate, magnesium oxide, methacrylic acid copolymer dispersion, mono and di glycerides, polysorbate 80, propylene glycol, shellac, sodium lauryl sulfate, strong ammonia solution, sugar spheres (which contains liquid glucose, starch (maize) and sucrose), talc, titanium dioxide, triethyl citrate and yellow iron oxide.
Questions or comments?
call 1 877 99 GENOM (43666) (Monday - Friday 8:30 AM to 5:00 PM EST)
Distributed by
Genomma Lab USA Inc., Houston, TX, 77027
Made in India
Code: TS/DRUGS/22/2009 -
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (14 Capsules Container Label)
Genozol®
Esomeprazole Magnesium / Magnesio de Esomeprazole
Delayed-Release Capsules USP 20 mg* /
Capsulas de liberacion prolongada USP 20 mg*
Acid Reducer / reductor de la acidez
Treats FREQUENT Heartburn!
May take 1 to 4 days for full effect
iAlivia el reflujo gastrico FRECUENTE!
Podria tardar entre 1 a 4 dias para lograr su accion completa
One 14-day courses of treatment
Un tratamientos de 14 dias
14 CAPSULES / CAPSULAS

-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 20 mg (14 Capsules Container Carton)
See new warning information / Ver la nueva información de advertencias
Genozol®
Esomeprazole Magnesium / Magnesio de Esomeprazole
Delayed-Release Capsules USP 20 mg* / Capsulas de liberacion prolongada USP 20 mg*
acid reducer / redutor de la acidez
Treats FREQUENT Heartburn!
May take 1 to 4 days for full effect
iAlivia el reflujo gastrico FRECUENTE!
Podria tardar entre 1 a 4 dias para lograr su accion complete
one 14-day course of treatment
Un tratamiento de 14 dias
14 CAPSULES / CAPSULAS
-
INGREDIENTS AND APPEARANCE
GENOZOL
esomeprazole magnesium capsule, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50066-923 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ESOMEPRAZOLE MAGNESIUM DIHYDRATE (UNII: 36H71644EQ) (ESOMEPRAZOLE - UNII:N3PA6559FT) ESOMEPRAZOLE 20 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM CARBONATE (UNII: 0E53J927NA) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) AMMONIA (UNII: 5138Q19F1X) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 14mm Flavor Imprint Code I81 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50066-923-05 1 in 1 CARTON 10/16/2017 1 14 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209339 10/16/2017 Labeler - Genomma Lab USA Inc (832323534) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650381903 ANALYSIS(50066-923) , MANUFACTURE(50066-923)
Trademark Results [GENOZOL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GENOZOL 97844735 not registered Live/Pending |
Genomma Lab Internacional, S.A.B de C.V. 2023-03-17 |
 GENOZOL 87021957 5188244 Live/Registered |
Genomma Lab Internacional, S.A.B. de C.V. 2016-05-02 |
 GENOZOL 86929122 5130410 Live/Registered |
Genomma Lab Internacional, S.A.B. de C.V. 2016-03-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.