GATIFLOXACIN solution/ drops
Gatifloxacin by
Drug Labeling and Warnings
Gatifloxacin by is a Prescription medication manufactured, distributed, or labeled by LUPIN LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Gatifloxacin Ophthalmic Solution, 0.5% safely and effectively. See full prescribing information for Gatifloxacin Ophthalmic Solution, 0.5%.
Gatifloxacin Ophthalmic Solution, 0.5%
Initial U.S. Approval: 1999INDICATIONS AND USAGE
Gatifloxacin ophthalmic solution, 0.5% is a topical fluoroquinolone anti-infective indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms:
Haemophilus influenzae, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus mitis group*, Streptococcus oralis*, Streptococcus pneumoniae
*Efficacy for this organism was studied in fewer than 10 infections. (1)
DOSAGE AND ADMINISTRATION
Patients 1 year of age or older: Instill one drop every two hours in the affected eye(s) while awake, up to 8 times on Day 1. Instill one drop two to four times daily in the affected eye(s) while awake on Days 2 through 7. (2)
DOSAGE FORMS AND STRENGTHS
5 mL size bottle filled with 2.5 mL of gatifloxacin ophthalmic solution, 0.5%. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions occurring in ≥ 1 % of patients included worsening of conjunctivitis, eye irritation, dysgeusia, and eye pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals, Inc. at 1-800-399-2561 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2016
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
5.2 Growth of Resistant Organisms with Prolonged Use
5.3 Avoidance of Contact Lens Wear
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Avoiding Contamination of the Product
17.2 Avoidance of Contact Lens Wear
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Gatifloxacin ophthalmic solution, 0.5% is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms:
Aerobic Gram-Positive Bacteria:
Staphylococcus aureus
Staphylococcus epidermidis
Streptococcus mitis group*
Streptococcus oralis*
Streptococcus pneumoniae
Aerobic Gram-Negative Bacteria:
Haemophilus influenzae
*Efficacy for this organism was studied in fewer than 10 infections.
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
Gatifloxacin ophthalmic solution, 0.5% should not be introduced directly into the anterior chamber of the eye.
5.2 Growth of Resistant Organisms with Prolonged Use
As with other anti-infectives, prolonged use of gatifloxacin ophthalmic solution, 0.5% may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit lamp biomicroscopy and where appropriate, fluoroscein staining.
5.3 Avoidance of Contact Lens Wear
Patients should be advised not to wear contact lenses if they have signs and symptoms of bacterial conjunctivitis or during the course of therapy with gatifloxacin ophthalmic solution, 0.5% (see PATIENT COUNSELING INFORMATION, 17.2).
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In clinical studies with gatifloxacin ophthalmic solution, 0.5% the most frequently reported adverse reactions occurring in ≥ 1 % of patients in the gatifloxacin study population (N=717) were: worsening of the conjunctivitis, eye irritation, dysgeusia, and eye pain.
Additional adverse events reported with other formulations of gatifloxacin ophthalmic solution include chemosis, conjunctival hemorrhage, dry eye, eye discharge, eyelid edema, headache, increased lacrimation, keratitis, papillary conjunctivitis, and reduced visual acuity.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: There were no teratogenic effects observed in rats or rabbits following oral gatifloxacin doses up to 50 mg/kg/day (approximately 1000-fold higher than the maximum recommended ophthalmic dose). However, skeletal/craniofacial malformations or delayed ossification, atrial enlargement, and reduced fetal weight were observed in fetuses from rats given ≥150 mg/kg/day (approximately 3000-fold higher than the maximum recommended ophthalmic dose). In a perinatal/postnatal study, increased late post-implantation loss and neonatal/perinatal mortalities were observed at 200 mg/kg/day (approximately 4000-fold higher than the maximum recommended ophthalmic dose).
Because there are no adequate and well-controlled studies in pregnant women, gatifloxacin ophthalmic solution, 0.5% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
Gatifloxacin is excreted in the breast milk of rats. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when gatifloxacin ophthalmic solution, 0.5% is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of gatifloxacin ophthalmic solution, 0.5% in infants below one year of age have not been established. Gatifloxacin ophthalmic solution, 0.5% has been demonstrated in clinical trials to be safe and effective for the treatment of bacterial conjunctivitis in pediatric patients one year or older (see CLINICAL STUDIES, 14).
-
11 DESCRIPTION
Gatifloxacin ophthalmic solution, 0.5% is an 8-methoxyfluoroquinolone anti-infective for the treatment of bacterial conjunctivitis. Its chemical name is (±) 1-Cyclopropyl-6-fluoro-8- methoxy-7-(3-methyl-1-piperazinyl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
Its molecular formula is C19H22FN3O4 and its molecular weight is 375.40. Its chemical structure is:
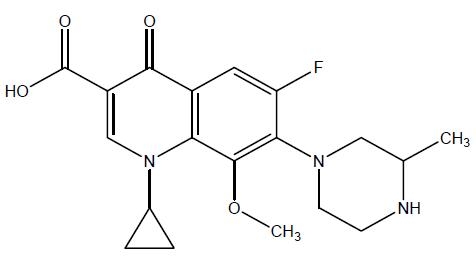
Gatifloxacin ophthalmic solution, 0.5% is a pale yellow colored transparent, sterile, liquid with an osmolality of 260 to 330 mOsm/kg and a pH of 5.1 to 5.7. Each mL of gatifloxacin ophthalmic solution, 0.5% contains Active: gatifloxacin 0.5% (5 mg) Preservative: benzalkonium chloride 0.005% Inactives: edetate disodium, water for injection and sodium chloride. May contain hydrochloric acid and/or sodium hydroxide to adjust pH.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Gatifloxacin is a fluoroquinolone antibacterial (see CLINICAL PHARMACOLOGY, 12.4).
12.3 Pharmacokinetics
Gatifloxacin ophthalmic solution 0.3% or 0.5% was administered to one eye of 6 healthy male subjects each in an escalated dosing regimen starting with a single 2 drop dose, then 2 drops 4 times daily for 7 days, and finally 2 drops 8 times daily for 3 days. At all time points, serum gatifloxacin levels were below the lower limit of quantification (5 ng/mL) in all subjects.
12.4 Microbiology
Gatifloxacin is an 8-methoxyfluoroquinolone with a 3-methylpiperazinyl substituent at C7. The antibacterial action of gatifloxacin results from inhibition of DNA gyrase and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription, and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division. The mechanism of action of fluoroquinolones including gatifloxacin is different from that of aminoglycoside, macrolide, and tetracycline antibiotics. Therefore, gatifloxacin may be active against pathogens that are resistant to these antibiotics and these antibiotics may be active against pathogens that are resistant to gatifloxacin. There is no cross-resistance between gatifloxacin and the aforementioned classes of antibiotics. Cross resistance has been observed between systemic gatifloxacin and some other fluoroquinolones.
Resistance to gatifloxacin in vitro develops via multiple-step mutations. Resistance to gatifloxacin in vitro occurs at a general frequency of 1 x 10-7 to 10-10.
Gatifloxacin has been shown to be active against most isolates of the following organisms both microbiologically and clinically, in conjunctival infections as described in the INDICATIONS AND USAGE, Section 1.
Aerobic Gram-Positive Bacteria:
Staphylococcus aureus
Staphylococcus epidermidis
Streptococcus mitis group*
Streptococcus oralis*
Streptococcus pneumoniae
Aerobic Gram-Negative Bacteria:
Haemophilus influenzae
*Efficacy for this organism was studied in fewer than 10 infections.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no increase in neoplasms among B6C3F1 mice given gatifloxacin in the diet for 18 months at doses averaging 81 mg/kg/day in males and 90 mg/kg/day in females. These doses are approximately 1600-fold and 1800-fold higher, respectively, than the maximum recommended ophthalmic dose of 0.05 mg/kg/day in a 50 kg human.
There was no increase in neoplasms among Fischer 344 rats given gatifloxacin in the diet for 2 years at doses averaging 47 mg/kg/day in males and 139 mg/kg/day in females (900- and 2800-fold higher, respectively, than the maximum recommended ophthalmic dose). A statistically significant increase in the incidence of large granular lymphocyte (LGL) leukemia was seen in males treated with a high dose of approximately 2000-fold higher than the maximum recommended ophthalmic dose. Fischer 344 rats have a high spontaneous background rate of LGL leukemia and the incidence in high-dose males only slightly exceeded the historical control range established for this strain.
In genetic toxicity tests, gatifloxacin was positive in 1 of 5 strains used in bacterial reverse mutation assays: Salmonella strain TA102. Gatifloxacin was positive in in vitro mammalian cell mutation and chromosome aberration assays. Gatifloxacin was positive in in vitro unscheduled DNA synthesis in rat hepatocytes but not human leukocytes. Gatifloxacin was negative in in vivo micronucleus tests in mice, cytogenetics test in rats, and DNA repair test in rats. The findings may be due to the inhibitory effects of high concentrations on eukaryotic type II DNA topoisomerase.
There were no adverse effects on fertility or reproduction in rats given gatifloxacin orally at doses up to 200 mg/kg/day (approximately 4000-fold higher than the maximum recommended ophthalmic dose for gatifloxacin ophthalmic solution, 0.5%).
-
14 CLINICAL STUDIES
In two randomized, double-masked, multicenter clinical trials, where patients 1-89 years of age were dosed for 5 days, gatifloxacin ophthalmic solution, 0.5% was clinically superior to its vehicle on day 6 in patients with conjunctivitis and positive conjunctival cultures. Clinical outcomes for the trials demonstrated clinical success (resolution of conjunctival hyperaemia and conjunctival discharge) of 58% (193/333) for the gatifloxacin-treated groups versus 45% (148/325) for the vehicle-treated groups. Microbiological outcomes for the same clinical trials demonstrated a statistically superior eradication rate for causative pathogens of 90% (301/333) for gatifloxacin versus 70% (228/325) for vehicle. Please note that microbiological eradication does not always correlate with clinical outcome in anti-infective trials.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Gatifloxacin Ophthalmic Solution, 0.5% is supplied sterile in a 5 mL white, low density polyethylene (LDPE) bottle fitted with a white low density polyethylene (LDPE) nozzle and sealed with a tan colored high density polyethylene (HDPE) cap in the following sizes:
2.5 mL in 5 mL bottle: NDC: 68180-435-01
Storage
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from freezing.
- 17 PATIENT COUNSELING INFORMATION
- SPL UNCLASSIFIED SECTION
-
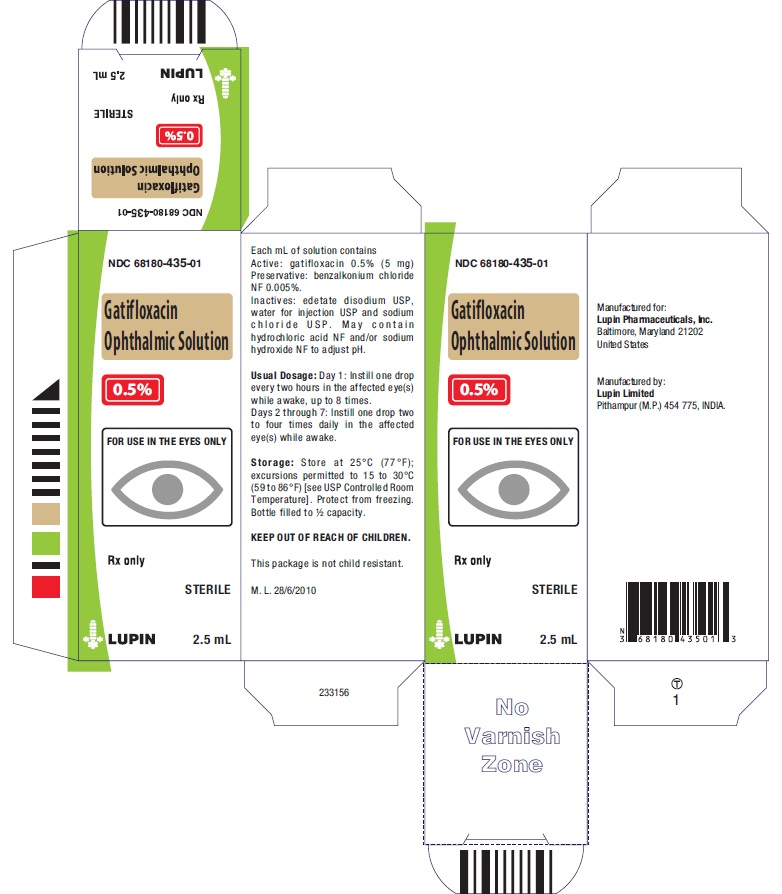
PRINCIPAL DISPLAY PANEL
GATIFLOXACIN OPHTHALMIC SOLUTION
Rx Only
0.5%
NDC: 68180-435-01
2.5 mL Bottle Label

GATIFLOXACIN OPHTHALMIC SOLUTION
Rx Only
0.5%
NDC: 68180-435-01
2.5 mL Carton Label
-
INGREDIENTS AND APPEARANCE
GATIFLOXACIN
gatifloxacin solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57297-435 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GATIFLOXACIN (UNII: L4618BD7KJ) (GATIFLOXACIN ANHYDROUS - UNII:81485Y3A9A) GATIFLOXACIN 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (PALE YELLOW) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57297-435-01 1 in 1 CARTON 10/01/2013 1 2.5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202653 10/01/2013 Labeler - LUPIN LIMITED (675923163) Registrant - LUPIN LIMITED (675923163) Establishment Name Address ID/FEI Business Operations LUPIN LIMITED 863645527 manufacture(57297-435) , pack(57297-435)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.